Thioacetanilide
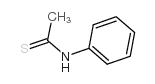
Thioacetanilide structure
|
Common Name | Thioacetanilide | ||
|---|---|---|---|---|
| CAS Number | 637-53-6 | Molecular Weight | 151.22900 | |
| Density | 1.16g/cm3 | Boiling Point | 147 °C / 1mmHg | |
| Molecular Formula | C8H9NS | Melting Point | 76-79 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 89.95ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
The metabolism of thioacetanilide in the rat.
Xenobiotica 13(8) , 475-82, (1983) The metabolism and acute toxicity of thioacetanilide was studied in the rat. Following intragastric dosage (100 mg/kg), over 90% dose was excreted in urine, predominantly as conjugated metabolites: less than 7% was recovered in the faeces, consisting of uncha... |
|
|
1,2,3-Selenadiazole thioacetanilides: synthesis and anti-HIV activity evaluation.
Bioorg. Med. Chem. 17(17) , 6374-9, (2009) The development of new HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) offers the possibility of generating novel structures of increased potency. Based on the bioisosteric principle, novel series of 1,2,3-selenadiazole thioacetanilide derivati... |
|
|
Synthesis and biological evaluation of imidazole thioacetanilides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors.
Bioorg. Med. Chem. 17(16) , 5775-81, (2009) A series of 2-(1-aryl-1H-imidazol-2-ylthio)acetamide [imidazole thioacetanilide (ITA)] derivatives were synthesized and evaluated as potent inhibitors of human immunodeficiency virus type-1 (HIV-1). Among them, the most potent HIV-1 inhibitors were 4a5 (EC(50... |
|
|
Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna.
Bioorg. Med. Chem. 20(18) , 5527-36, (2012) Relatively little is known about the behavior and toxicity of nanoparticles in the environment. Objectives of work presented here include establishing the toxicity of a variety of silver nanoparticles (AgNPs) to Daphnia magna neonates, assessing the applicabi... |
|
|
[Substantiation of maximum permissible levels of thioacylanilide in the air of work areas].
Gig. Tr. Prof. Zabol. (7) , 51-2, (1986)
|
|
|
Application of beta-(2-chloroaroyl) thioacetanilides in synthesis: an unusual and highly efficient access to thiochromeno[2,3-b]pyridine derivatives.
J. Org. Chem. 73(5) , 1852-63, (2008) A series of unusual fused tricyclic thiochromeno[2,3-b]pyridines were successfully synthesized by tandem [3 + 3] annulation and SNAr of beta-(2-chloroaroyl) thioacetanilides with activated 4-arylidene-2-phenyloxazol-5(4H)-ones or aromatic aldehydes and ethyl ... |
|
|
Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103N mutant.
Bioorg. Med. Chem. Lett. 16(10) , 2748-52, (2006) A series of aryltetrazolylacetanilides was synthesized and evaluated as HIV-1 non-nucleoside reverse transcriptase inhibitors on wild-type virus and on the clinically relevant K103N mutant strain. Extensive SAR investigation led to potent compounds, with nano... |
|
|
Optical analysis of the cirrhotic liver by near-infrared time-resolved spectroscopy.
Surg. Today 34(5) , 424-8, (2004) To assess the histological severity of liver cirrhosis in relation to the optical properties of liver tissue.Various grades of liver cirrhosis were induced in rats by giving intraperitoneal injections of thioacetamide (TAA) over periods ranging from 4 to 16 w... |
|
|
Thioacetanilide at 120 K.
Acta Crystallogr. C 64(Pt 8) , o411-3, (2008) The title compound, C(8)H(9)NS, has four symmetry-independent molecules in the asymmetric unit. These molecules link into two independent infinite N-H...S hydrogen-bonded chains in the a-axis direction with graph-set notation C(2)(2)(8). The NH-CS group adopt... |
|
|
Reactivity of superoxide ion with thioamides in dimethyl sulfoxide. Paez OA, et al.
J. Org. Chem. 53(10) , 2166-70, (1988)
|