1,2,3-Selenadiazole thioacetanilides: synthesis and anti-HIV activity evaluation.
Peng Zhan, Xinyong Liu, Zengjun Fang, Christophe Pannecouque, Erik De Clercq
Index: Bioorg. Med. Chem. 17(17) , 6374-9, (2009)
Full Text: HTML
Abstract
The development of new HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) offers the possibility of generating novel structures of increased potency. Based on the bioisosteric principle, novel series of 1,2,3-selenadiazole thioacetanilide derivatives were designed, and synthesized using an original synthetic route, structurally confirmed by spectral analysis, and evaluated for their anti-HIV activity in MT-4 cells. The results showed that only compound 7f possessed potent activity against HIV-1 replication (EC(50)=2.45 microM) similar to the prototype series of sulfanyltriazoles. None of the compounds were active against HIV-2 replication.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
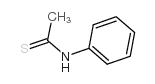 |
Thioacetanilide
CAS:637-53-6 |
C8H9NS |
|
The metabolism of thioacetanilide in the rat.
1983-08-01 [Xenobiotica 13(8) , 475-82, (1983)] |
|
Synthesis and biological evaluation of imidazole thioacetani...
2009-08-15 [Bioorg. Med. Chem. 17(16) , 5775-81, (2009)] |
|
Effects from filtration, capping agents, and presence/absenc...
2010-12-01 [Bioorg. Med. Chem. 20(18) , 5527-36, (2012)] |
|
[Substantiation of maximum permissible levels of thioacylani...
1986-07-01 [Gig. Tr. Prof. Zabol. (7) , 51-2, (1986)] |
|
Application of beta-(2-chloroaroyl) thioacetanilides in synt...
2008-03-07 [J. Org. Chem. 73(5) , 1852-63, (2008)] |