4-Bromobenzylamine
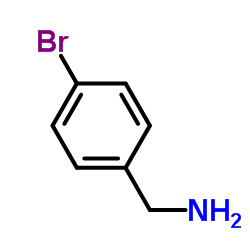
4-Bromobenzylamine structure
|
Common Name | 4-Bromobenzylamine | ||
|---|---|---|---|---|
| CAS Number | 3959-07-7 | Molecular Weight | 186.049 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 248.9±15.0 °C at 760 mmHg | |
| Molecular Formula | C7H8BrN | Melting Point | 25 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 104.3±20.4 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism.
Proc. Natl. Acad. Sci. U. S. A. 111(23) , 8428-33, (2014) Voltage-gated sodium channels are important targets for the development of pharmaceutical drugs, because mutations in different human sodium channel isoforms have causal relationships with a range of neurological and cardiovascular diseases. In this study, fu... |
|
|
Common metal of copper(0) as an efficient catalyst for preparation of nitriles and imines by controlling additives.
Chem. Commun. (Camb.) 50(42) , 5637-40, (2014) A novel, efficient, convenient and environmentally friendly approach for the synthesis of nitriles and imines from primary amines has been developed. Using commercially available red copper as the catalyst, ammonium bromide as the co-catalyst and molecular ox... |
|
|
Novel series of substituted biphenylmethyl urea derivatives as MCH-R1 antagonists for the treatment of obesity.
Bioorg. Med. Chem. 15(11) , 3896-911, (2007) We have designed and synthesized two novel series of MCH-R1 antagonists based on a substituted biphenylmethyl urea core. SAR was explored, suggesting that optimal binding with the receptor was achieved when the biphenylmethyl group and the linker were substit... |
|
|
Postsynthetic modification of peptoids via the Suzuki-Miyaura cross-coupling reaction.
Biopolymers 106 , 82-8, (2016) We developed a new method for modifying the side chains of peptoids on a solid phase resin, employing the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Optimized conditions using Pd(PPh3 )4 and K2 CO3 in the presence of Buchwald's SPhos ligand p... |
|
|
Efficient synthesis of 2,6,9-triazabicyclo [3.3.1] nonanes through amine-mediated formal [4+4] reaction of unsaturated imines. Tanaka K, et al.
Tetrahedron Lett. 53(44) , 5899-5902, (2012)
|
|
|
Nitrogenous bisabolene sesquiterpenes from marine invertebrates. Gulavita NK, et al.
J. Org. Chem. 51(26) , 5136-5139, (1986)
|