Bioorganic & Medicinal Chemistry
2007-06-01
Novel series of substituted biphenylmethyl urea derivatives as MCH-R1 antagonists for the treatment of obesity.
Silvia Galiano, Javier Ceras, Nuria Cirauqui, Silvia Pérez, Laura Juanenea, Gildardo Rivera, Ignacio Aldana, Antonio Monge
Index: Bioorg. Med. Chem. 15(11) , 3896-911, (2007)
Full Text: HTML
Abstract
We have designed and synthesized two novel series of MCH-R1 antagonists based on a substituted biphenylmethyl urea core. SAR was explored, suggesting that optimal binding with the receptor was achieved when the biphenylmethyl group and the linker were substituted on the same nitrogen of the urea moiety. Compound 1-(3'-cyano-4-biphenylmethyl)-3-(2-hydroxy-1,1-dimethylethyl)-1-{2-[1-(4-methylbenzyl)-4-piperidinyl]ethyl}urea 2t showed the best antagonist binding activity to the MCH-R1 with a 43 nM K(i).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
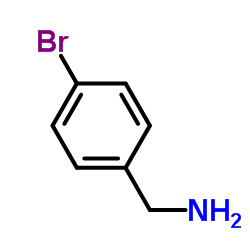 |
4-Bromobenzylamine
CAS:3959-07-7 |
C7H8BrN |
Related Articles:
More...
|
Prokaryotic NavMs channel as a structural and functional mod...
2014-06-10 [Proc. Natl. Acad. Sci. U. S. A. 111(23) , 8428-33, (2014)] |
|
Common metal of copper(0) as an efficient catalyst for prepa...
2014-05-30 [Chem. Commun. (Camb.) 50(42) , 5637-40, (2014)] |
|
Postsynthetic modification of peptoids via the Suzuki-Miyaur...
2016-01-01 [Biopolymers 106 , 82-8, (2016)] |
|
Efficient synthesis of 2,6,9-triazabicyclo [3.3.1] nonanes t...
[Tetrahedron Lett. 53(44) , 5899-5902, (2012)] |
|
Nitrogenous bisabolene sesquiterpenes from marine invertebra...
[J. Org. Chem. 51(26) , 5136-5139, (1986)] |