trifluoromethanesulfonimide
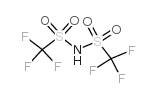
trifluoromethanesulfonimide structure
|
Common Name | trifluoromethanesulfonimide | ||
|---|---|---|---|---|
| CAS Number | 82113-65-3 | Molecular Weight | 281.15400 | |
| Density | 1.936 g/cm3 | Boiling Point | 90-91 °C(lit.) | |
| Molecular Formula | C2HF6NO4S2 | Melting Point | 52-56 °C | |
| MSDS | Chinese USA | Flash Point | 69ºC | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Bis (trifluoromethane) sulfonimide initiated ring-opening polymerization of octamethylcyclotetrasiloxane. Desmurs J-R, et al.
J. Organomet. Chem. 646(1) , 171-8, (2002)
|
|
|
Comparative Electrochemical Study of New Poly(oxyethylene)-Li Salt Complexes. Benrabah D, et al.
J. Chem. Soc., Faraday 89 , 355-359, (1993)
|
|
|
Synthesis of trehalose mimics by bismuth (III) triflate or bis (trifluoromethane) sulfonimide-catalyzed 1-C-methyl-D-hexopyranosylation. Yamanoi T, et al.
Tetrahedron Asymmetry 17(20) , 2914-2918, (2006)
|
|
|
Self-assembly of cationic rod-like poly (2, 5-pyridine) by acidic bis (trifluoromethane) sulfonimide in the hydrated state: A highly-ordered self-assembled protonic conductor. Vilkman M, et al.
Polymer 51(18) , 4095-4102, (2010)
|
|
|
Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Bernardo P, et al.
Separ. Purif. Tech. 97 , 73-82, (2012)
|
|
|
N-Fluorobis[(perfluoroalkyl)sulfonyl]imides: Reactions with some olefins via α-fluoro carbocationic intermediates. Desmarteau DD, et al.
J. Org. Chem. 57(2) , 629-635, (1992)
|
|
|
Synlett , 171, (1996)
|
|
|
IR spectra of trifluoromethanesulfonamide and its self-associates in the gas phase. Chipanina NN, et al.
Russ. J. Gen. Chem. 74(4) , 582-585, (2004)
|
|
|
Cascade transformations of trifluoromethanesulfonamide in reaction with formaldehyde. Meshcheryakov VI, et al.
Russ. J. Gen. Chem. 41(9) , 1381-1386, (2005)
|
|
|
Infrared and Raman spectra and ab initio calculations for normal and deuterated trifluoromethanesulfonamide. Fernandez LE, et al.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 53(2) , 189-197, (1997)
|