2-Methyl-indanone
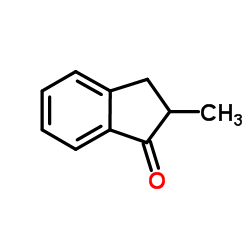
2-Methyl-indanone structure
|
Common Name | 2-Methyl-indanone | ||
|---|---|---|---|---|
| CAS Number | 17496-14-9 | Molecular Weight | 146.186 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 238.4±10.0 °C at 760 mmHg | |
| Molecular Formula | C10H10O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 94.4±14.0 °C | |
|
The O-acylation of ketone enolates by allyl 1H-imidazole-1-carboxylate mediated with boron trifluoride etherate: a convenient procedure for the synthesis of substituted allyl enol carbonates.
J. Org. Chem. 72(24) , 9372-5, (2007) A convenient access to substituted allyl enol carbonates was established through the reaction of ketone enolates with the complex of allyl 1H-imidazole-1-carboxylates and boron trifluoride etherate. |
|
|
Recent advances in enzymatic and chemical deracemisation of racemic compounds.
Chem. Soc. Rev. 42(24) , 9268-9282, (2013) Deracemisation of racemic compounds is still the most important strategy to produce optically pure compounds despite many recent advances in asymmetric synthesis. Especially deracemisation approaches that give rise to single enantiomers are preferred, which c... |
|
|
Amino alcohol-mediated enantioselective syntheses of α-substituted indanones and tetralones, ammonium enolates as key intermediates. Muzart J.
Tetrahedron Asymmetry 25(9) , 697-704, (2014)
|
|
|
Structural effects in the Pd-induced enantioselective deprotection-decarboxylation of β-ketoesters. Kukula P, et al.
Tetrahedron Asymmetry 18(24) , 2859-2868, (2007)
|
|
|
Heck-type reactions of allylic alcohols: Part IV:(2-Substituted)-1-indanones via 5-endo-trig cyclizations. Zawisza AM, et al.
J. Mol. Catal. A: Chem. 283(1) , 140-45, (2008)
|
|
|
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and charge-transfer interaction. Luhowy R and Keehn PM.
J. Am. Chem. Soc. 99(11) , 3797-3805, (1977)
|
|
|
On the decarboxylation of 2-methyl-1-tetralone-2-carboxylic acid-oxidation of the enol intermediate by triplet oxygen. Riahi A, et al.
New J. Chem. 37(8) , 2245-2249, (2013)
|
|
|
Selective and easy preparation of enol carbonates of α-disubstituted aryl ketones from their lithium enolates. Aboulhoda SJ, et al.
Tetrahedron Lett. 36(27) , 4795-4796, (1995)
|
|
|
Toward chemistry-based design of the simplest metalloenzyme-like catalyst that works efficiently in water.
Chem. Asian J. 10(1) , 133-138, (2015) Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of hars... |
|
|
Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes: effect of halide on selectivity, oxidation state, and room-temperature reactions.
J. Am. Chem. Soc. 133 , 16330, (2011) We report the α-arylation of ketones with a range of aryl chlorides with enantioselectivities from 90 to 99% ee catalyzed by the combination of Ni(COD)(2) and (R)-BINAP and the coupling of ketones with a range of heteroaryl chlorides with enantioselectivities... |