Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes: effect of halide on selectivity, oxidation state, and room-temperature reactions.
Shaozhong Ge, John F Hartwig
Index: J. Am. Chem. Soc. 133 , 16330, (2011)
Full Text: HTML
Abstract
We report the α-arylation of ketones with a range of aryl chlorides with enantioselectivities from 90 to 99% ee catalyzed by the combination of Ni(COD)(2) and (R)-BINAP and the coupling of ketones with a range of heteroaryl chlorides with enantioselectivities up to 99% ee catalyzed by Ni(COD)(2) and (R)-DIFLUORPHOS. The analogous reactions of bromoarenes occur with much lower enantioselectivities. Mechanistic studies showed that the difference in the rates of decomposition of the arylnickel(II) halide intermediates to {[(R)-BINAP]NiX}(2) likely accounts for the difference in the enantioselectivities of the reactions of bromoarenes and chloroarenes. This catalyst decomposition can be overcome by conducting the reactions with [(R)-BINAP]Ni(η(2)-NC-Ph) (4), which undergoes oxidative addition to haloarenes at room temperature.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
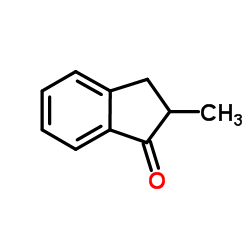 |
2-Methyl-indanone
CAS:17496-14-9 |
C10H10O |
|
The O-acylation of ketone enolates by allyl 1H-imidazole-1-c...
2007-11-23 [J. Org. Chem. 72(24) , 9372-5, (2007)] |
|
Recent advances in enzymatic and chemical deracemisation of ...
2013-12-21 [Chem. Soc. Rev. 42(24) , 9268-9282, (2013)] |
|
Amino alcohol-mediated enantioselective syntheses of α-...
[Tetrahedron Asymmetry 25(9) , 697-704, (2014)] |
|
Structural effects in the Pd-induced enantioselective deprot...
[Tetrahedron Asymmetry 18(24) , 2859-2868, (2007)] |
|
Heck-type reactions of allylic alcohols: Part IV:(2-Substitu...
[J. Mol. Catal. A: Chem. 283(1) , 140-45, (2008)] |