Bakuchiol
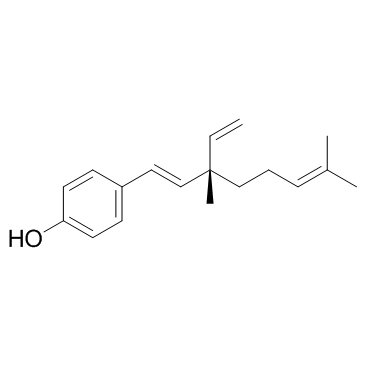
Bakuchiol structure
|
Common Name | Bakuchiol | ||
|---|---|---|---|---|
| CAS Number | 10309-37-2 | Molecular Weight | 256.383 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 391.4±21.0 °C at 760 mmHg | |
| Molecular Formula | C18H24O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 176.6±11.7 °C | |
|
Bakuchiol derivatives as novel and potent cytotoxic agents: a report.
Eur. J. Med. Chem. 49C , 55-67, (2012) A library of 28 compounds comprising of acyl, amino, halo, nitro, styryl and cyclized derivatives of bakuchiol have been evaluated against a panel of eight human cancer cell lines. Bioevaluation studies have resulted in the identification of potent cytotoxic ... |
|
|
Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation.
Planta Med. 78(9) , 903-6, (2012) Inhibiting interleukin-6 (IL-6) has been postulated as an effective therapy in the pathogenesis of several inflammatory diseases. In this study, seven flavonoids were isolated from the methanol extracts of Psoralea corylifolia by bioactivity-guided fractionat... |
|
|
Chemical fingerprint and quantitative analysis of fructus psoraleae by high-performance liquid chromatography.
J. Sep. Sci. 30(6) , 813-8, (2007) Fructus Psoraleae, a widely used traditional Chinese medicine, is well known as a health supplement ingredient. In our study, an improved and comprehensive HPLC fingerprint of Fructus Psoraleae was established. Two important new benzofuran glycosides, psorale... |
|
|
Two antifungal components isolated from Fructus Psoraleae and Folium Eucalypti Globuli by bioassay-guided purification.
Am. J. Chin. Med. 38(5) , 1005-14, (2010) Fructus Psoraleae and Folium Eucalypti Globuli have long been used as Chinese medicines to treat various ailments such as asthma, eczema and dermatomycosis. In previous studies, their antifungal activities were demonstrated. The aim of the present study was t... |
|
|
In vivo pharmacokinetics of bakuchiol after oral administration of bakuchiol extraction in rat plasma.
J. Ethnopharmacol. 128(3) , 697-702, (2010) Psoralea corylifolia L. (Fabaceae) was a traditional Chinese medicine for the treatment of various skin diseases such as psoriasis, vitiligo and chronic graft-versus-host, and has been proved to show anticancer, cytotoxic, anti-bacterial, cardiac, diaphoretic... |
|
|
Synthesis and structure-immunosuppressive activity relationships of bakuchiol and its derivatives.
Bioorg. Med. Chem. 16(5) , 2403-11, (2008) A series of derivatives of bakuchiol were synthesized and tested in vitro for their cytotoxicity, and inhibition of T cell proliferation and B cell proliferation. The data obtained provided preliminary structure-activity relationships of the compounds as immu... |
|
|
Novel bisstyryl derivatives of bakuchiol: Targeting oral cavity pathogens
Eur. J. Med. Chem. 45 , 3125-34, (2010) Novel bisstyryl derivatives of bakuchiol using Heck coupling reaction as the key step were synthesized and screened against a panel of six oral cavity pathogens for their antimicrobial activity. Four compounds ( 9– 12) showed two to fourfold and four to eight... |
|
|
Tumor cell death induced by the inhibition of mitochondrial electron transport: the effect of 3-hydroxybakuchiol.
Toxicol. Appl. Pharmacol. 272(2) , 356-64, (2013) Changes in mitochondrial ATP synthesis can affect the function of tumor cells due to the dependence of the first step of glycolysis on mitochondrial ATP. The oxidative phosphorylation (OXPHOS) system is responsible for the synthesis of approximately 90% of th... |
|
|
Hypoxia-inducible factor-1 and nuclear factor-kappaB inhibitory meroterpene analogues of bakuchiol, a constituent of the seeds of Psoralea corylifolia.
Bioorg. Med. Chem. Lett. 18(8) , 2619-23, (2008) Two new meroterpenoids, 12,13-dihydro-12,13-dihydroxybakuchiol (2) and (12'S)-bisbakuchiol C (3), were isolated from the seeds of Psoralea corylifolia L. (Fabaceae). The structures of 2 and 3 were elucidated by spectroscopic and chemical methods. Six meroterp... |
|
|
Asymmetric construction of all-carbon quaternary stereocenters by chiral-auxiliary-mediated Claisen rearrangement and total synthesis of (+)-bakuchiol.
Molecules 17(11) , 13330-44, (2012) An asymmetric Claisen rearrangement using Oppolzer’s camphorsultam was developed. Under thermal conditions, a geraniol-derived substrate underwent the rearrangement with good stereoselectivity. The absolute configuration of the newly formed all-carbon quatern... |