Molecules
2012-01-01
Asymmetric construction of all-carbon quaternary stereocenters by chiral-auxiliary-mediated Claisen rearrangement and total synthesis of (+)-bakuchiol.
Ken-ichi Takao, Shu Sakamoto, Marianne Ayaka Touati, Yusuke Kusakawa, Kin-ichi Tadano
Index: Molecules 17(11) , 13330-44, (2012)
Full Text: HTML
Abstract
An asymmetric Claisen rearrangement using Oppolzer’s camphorsultam was developed. Under thermal conditions, a geraniol-derived substrate underwent the rearrangement with good stereoselectivity. The absolute configuration of the newly formed all-carbon quaternary stereocenter was confirmed by the total synthesis of (+)-bakuchiol from the rearrangement product.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
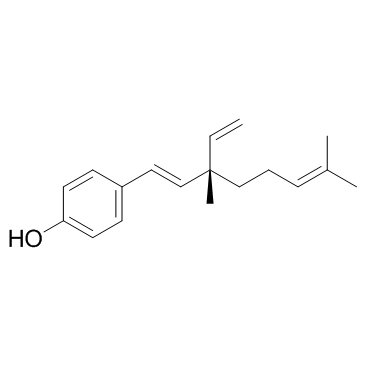 |
Bakuchiol
CAS:10309-37-2 |
C18H24O |
Related Articles:
More...
|
Bakuchiol derivatives as novel and potent cytotoxic agents: ...
2012-03-01 [Eur. J. Med. Chem. 49C , 55-67, (2012)] |
|
Phenolic compounds isolated from Psoralea corylifolia inhibi...
2012-06-01 [Planta Med. 78(9) , 903-6, (2012)] |
|
Chemical fingerprint and quantitative analysis of fructus ps...
2007-04-01 [J. Sep. Sci. 30(6) , 813-8, (2007)] |
|
Two antifungal components isolated from Fructus Psoraleae an...
2010-01-01 [Am. J. Chin. Med. 38(5) , 1005-14, (2010)] |
|
In vivo pharmacokinetics of bakuchiol after oral administrat...
2010-04-21 [J. Ethnopharmacol. 128(3) , 697-702, (2010)] |