H-Cys(Bzl)-OH
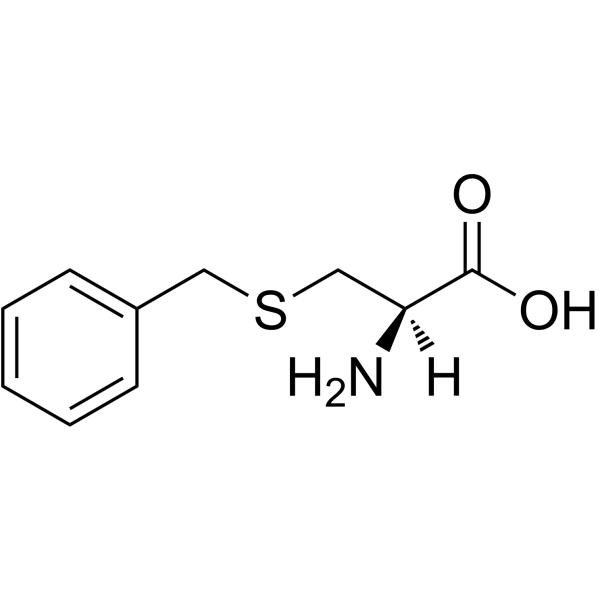
H-Cys(Bzl)-OH structure
|
Common Name | H-Cys(Bzl)-OH | ||
|---|---|---|---|---|
| CAS Number | 3054-01-1 | Molecular Weight | 211.281 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 379.2±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H13NO2S | Melting Point | 209-214ºC | |
| MSDS | Chinese USA | Flash Point | 183.2±27.9 °C | |
|
Comparison of peak-picking workflows for untargeted liquid chromatography/high-resolution mass spectrometry metabolomics data analysis.
Rapid Commun. Mass Spectrom. 29(1) , 119-27, (2014) Data analysis is a key step in mass spectrometry based untargeted metabolomics, starting with the generation of generic peak lists from raw liquid chromatography/mass spectrometry (LC/MS) data. Due to the use of various algorithms by different workflows, the ... |
|
|
Formation of a dehydroalanyl residue from S-benzylcysteine upon HF cleavage of a [Sar1, Cys8]-angiotensin II peptide resin.
Int. J. Pept. Protein Res. 38(6) , 601-2, (1991)
|
|
|
Synthesis and biological evaluation of L-cysteine derivatives as mitotic kinesin Eg5 inhibitors.
Bioorg. Med. Chem. Lett. 17 , 3921-4, (2007) Inhibition of Eg5 represents a novel approach for the treatment of cancer. Here, we report the synthesis and structure-activity relationship of S-trityl-L-cysteine (STLC) derivatives as Eg5 inhibitors. Some of these derivatives such as 4f demonstrated enhance... |
|
|
Role of allyl group in the hydroxyl and peroxyl radical scavenging activity of S-allylcysteine.
J. Phys. Chem. B 115(45) , 13408-17, (2011) S-Allylcysteine (SAC) is the most abundant compound in aged garlic extracts, and its antioxidant properties have been demonstrated. It is known that SAC is able to scavenge different reactive species including hydroxyl radical (•OH), although its potential ab... |
|
|
Localization and capacity of the last step of mercapturic acid biosynthesis and the reabsorption and acetylation of cysteine S-conjugates in the rat kidney.
Pflugers Arch. 417(5) , 523-7, (1991) We investigated the capacity and the localization of N-acetylation of the mercapturic acid precursor S-benzyl-L-cysteine (BC), as well as the tubular reabsorption of this compound in the rat kidney in vivo et situ by renal clearance and continuous microinfusi... |
|
|
Cysteine, a chelating moiety for synthesis of technetium-99m radiopharmaceuticals--Part IV. Benzyl cysteine and derivatives.
Nucl. Med. Biol. 20(4) , 413-26, (1993) To explore the possibility of utilizing cysteine derivatives for technetium-99m radiopharmaceutical preparation with clinical potential, we synthesized two benzyl substituted cysteine compounds, namely, S-benzyl cysteine 1 and cysteine benzyl ester 3. It was ... |
|
|
Mercapturic acid formation in cultured opossum kidney cells.
Toxicol. Lett. 53(1-2) , 261, (1990)
|
|
|
Mercapturic acid formation in the marmoset (Callithrix jacchus).
Xenobiotica 16(7) , 609-14, (1986) Benzylmercapturic acid is a major metabolite of [methylene-14C]benzyl chloride in the marmoset, as in the rat. The excretion of the minor metabolites benzylmercapturic acid sulphoxide and benzylcysteine accounted for a greater proportion of the dose than in t... |
|
|
Selenoxidation by flavin-containing monooxygenases as a novel pathway for beta-elimination of selenocysteine Se-conjugates.
Chem. Res. Toxicol. 14(1) , 127-34, (2001) Previously, it was shown that beta-elimination of selenocysteine Se-conjugates by rat renal cytosol leading to pyruvate formation was not solely catalyzed by pyridoxal phosphate-dependent enzymes. It was hypothesized that selenoxidation of the selenocysteine ... |
|
|
A potent pyridoxal model capable of promoting transamination and beta-elimination of amino acids.
Prog. Clin. Biol. Res. 144A , 101-8, (1984)
|