H-Cys(Bzl)-OH
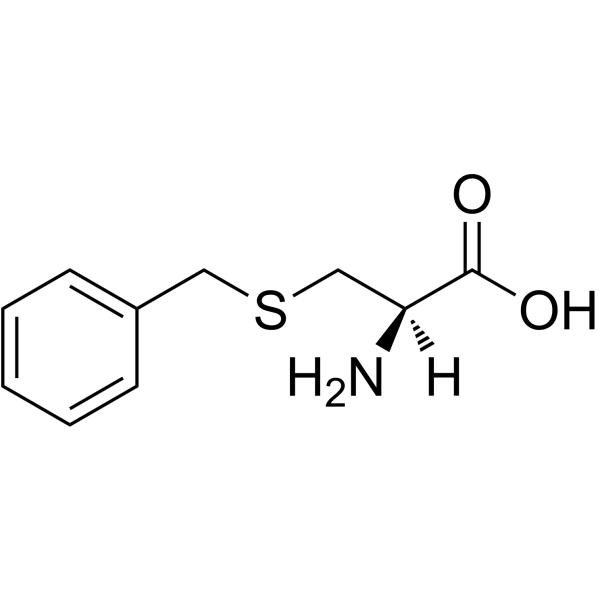
H-Cys(Bzl)-OH structure
|
Common Name | H-Cys(Bzl)-OH | ||
|---|---|---|---|---|
| CAS Number | 3054-01-1 | Molecular Weight | 211.281 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 379.2±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H13NO2S | Melting Point | 209-214ºC | |
| MSDS | Chinese USA | Flash Point | 183.2±27.9 °C | |
Use of H-Cys(Bzl)-OHH-Cys(Bzl)-OH is a cysteine derivative[1]. |
| Name | (R)-S-Benzylcysteine |
|---|---|
| Synonym | More Synonyms |
| Description | H-Cys(Bzl)-OH is a cysteine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 379.2±42.0 °C at 760 mmHg |
| Melting Point | 209-214ºC |
| Molecular Formula | C10H13NO2S |
| Molecular Weight | 211.281 |
| Flash Point | 183.2±27.9 °C |
| Exact Mass | 211.066696 |
| PSA | 88.62000 |
| LogP | 2.10 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Comparison of peak-picking workflows for untargeted liquid chromatography/high-resolution mass spectrometry metabolomics data analysis.
Rapid Commun. Mass Spectrom. 29(1) , 119-27, (2014) Data analysis is a key step in mass spectrometry based untargeted metabolomics, starting with the generation of generic peak lists from raw liquid chromatography/mass spectrometry (LC/MS) data. Due to... |
|
|
Formation of a dehydroalanyl residue from S-benzylcysteine upon HF cleavage of a [Sar1, Cys8]-angiotensin II peptide resin.
Int. J. Pept. Protein Res. 38(6) , 601-2, (1991)
|
|
|
Synthesis and biological evaluation of L-cysteine derivatives as mitotic kinesin Eg5 inhibitors.
Bioorg. Med. Chem. Lett. 17 , 3921-4, (2007) Inhibition of Eg5 represents a novel approach for the treatment of cancer. Here, we report the synthesis and structure-activity relationship of S-trityl-L-cysteine (STLC) derivatives as Eg5 inhibitors... |
| Alanine, 3- (benzylthio)-, L- |
| D-S-Benzylcysteine |
| EINECS 221-273-4 |
| MFCD00002613 |
| H-CYS(BZL)-OH |
| H-L-CYS(BZL)-OH |
| S-Benzyl-L-Cysteine |
| Benzylcysteine |
| (L)-2-amino-3-(benzylthio)propanoic acid |
| (S)-BENZYL-L-CYS |
| (S)-S-benzylcysteine |
| L-Cysteine, S- (phenylmethyl)- |
| L-S-benzylcysteine |
| (R)-2-Amino-3- (S-Benzylthio)Propanoic Acid |
| S-Benzylcysteine |
| BENZYL-S-CYSTEINE |
| (R)-2-Amino-3-(benzylthio)propanoic acid |
| Cysteine, S-(phenylmethyl)- |
| S-Bzl-L-Cys |
| CYSTEINE(BZL)-OH |
 CAS#:52-89-1
CAS#:52-89-1 CAS#:100-39-0
CAS#:100-39-0 CAS#:100-44-7
CAS#:100-44-7 CAS#:52-90-4
CAS#:52-90-4 CAS#:56-45-1
CAS#:56-45-1 CAS#:100-53-8
CAS#:100-53-8 CAS#:56-89-3
CAS#:56-89-3 CAS#:2731-73-9
CAS#:2731-73-9![L-Cysteine,N-[(4-methylphenyl)sulfonyl]-S-(phenylmethyl)- Structure](https://image.chemsrc.com/caspic/105/4703-36-0.png) CAS#:4703-36-0
CAS#:4703-36-0 CAS#:196930-46-8
CAS#:196930-46-8 CAS#:5068-28-0
CAS#:5068-28-0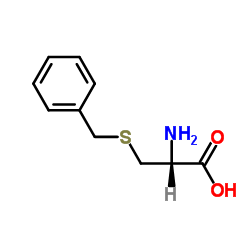 CAS#:3257-18-9
CAS#:3257-18-9 CAS#:495-69-2
CAS#:495-69-2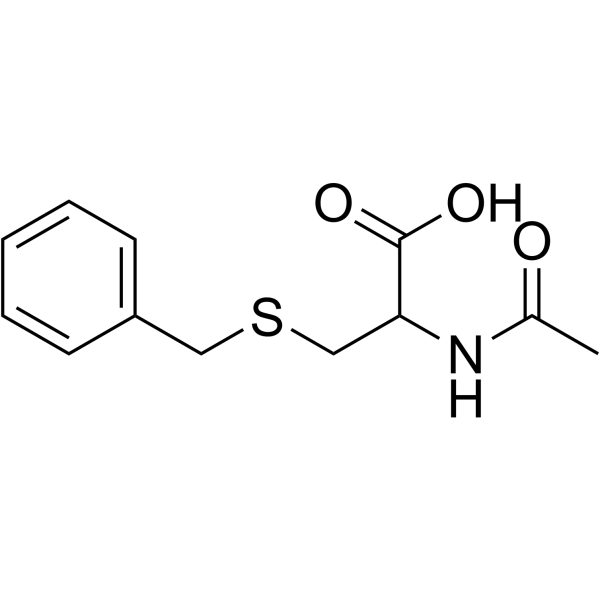 CAS#:19538-71-7
CAS#:19538-71-7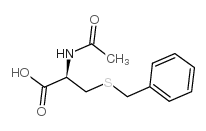 CAS#:19542-77-9
CAS#:19542-77-9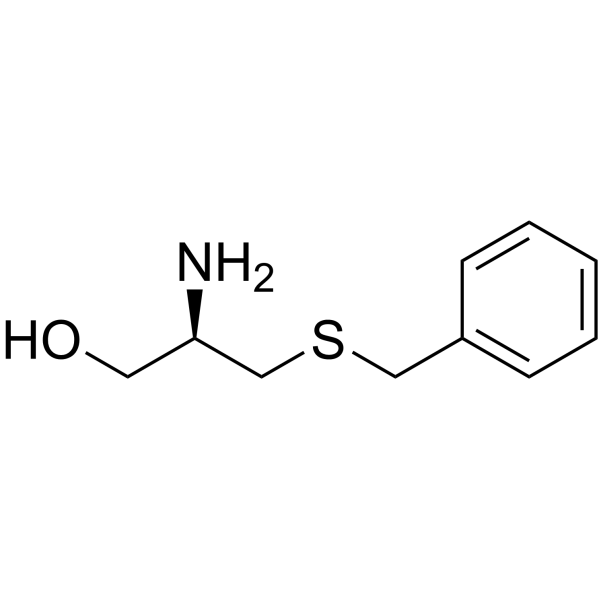 CAS#:85803-43-6
CAS#:85803-43-6 CAS#:86060-95-9
CAS#:86060-95-9 CAS#:53298-33-2
CAS#:53298-33-2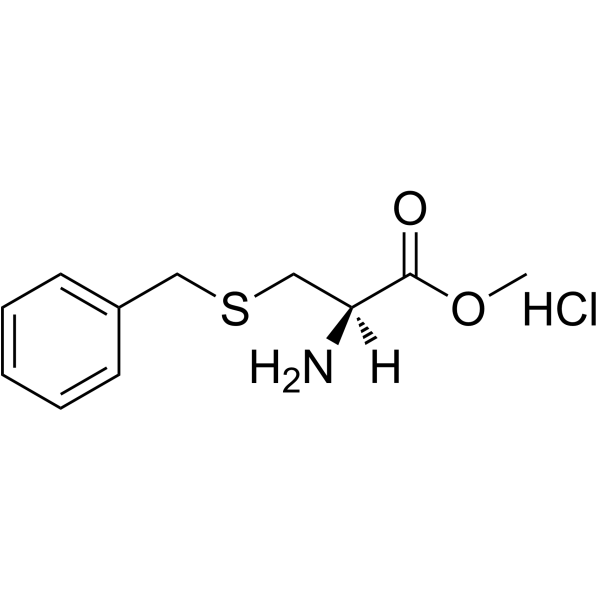 CAS#:16741-80-3
CAS#:16741-80-3 CAS#:139428-96-9
CAS#:139428-96-9
