N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE
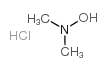
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE structure
|
Common Name | N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE | ||
|---|---|---|---|---|
| CAS Number | 16645-06-0 | Molecular Weight | 97.54400 | |
| Density | N/A | Boiling Point | 64.2ºC at 760mmHg | |
| Molecular Formula | C2H8ClNO | Melting Point | 107-109ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 16.5ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
In vitro haematotoxic effects of three methylated hydroxylamines.
Arch. Toxicol. 71(5) , 299-305, (1997) Hydroxylamine (HYAM, HONH2) and some of its derivatives are known to cause erythrotoxic effects both in vitro and in vivo. Previous studies have shown that the primary in vitro effect of HYAM and O-ethyl hydroxylamine (OEH) is methaemoglobin formation, leadin... |
|
|
Free radical intermediates in the oxidation of N-methylhydroxylamine and N,N-dimethylhydroxylamine by oxyhemoglobin.
Free Radic. Res. Commun. 8(2) , 123-31, (1990) Nitroxide radicals have been detected in the methemoglobin formation reaction between oxyhemoglobin and the substituted hydroxylamine compounds, N-methylhydroxylamine and N,N-dimethylhydroxylamine, by ESR spectroscopy. The stability of these nitroxide radical... |
|
|
Two mechanisms for toxic effects of hydroxylamines in human erythrocytes: involvement of free radicals and risk of potentiation.
Blood Cells Mol. Dis. 24(3) , 280-95, (1998) The toxic potency of three industrially used hydroxylamines was studied in human blood cells in vitro. The parent compound hydroxylamine and the O-ethyl derivative gave very similar results. Both compounds induced a high degree of methemoglobin formation and ... |
|
|
A one-flask synthesis of Weinreb amides from chiral and achiral carboxylic acids using the deoxo-fluor fluorinating reagent.
Org. Lett. 2(25) , 4091-3, (2000) [structure] The reagent [bis(2-methoxyethyl)amino]sulfur trifluoride (Deoxo-Fluor reagent) converts carboxylic acids to the corresponding acid fluorides, which then react with N,N-dimethylhydroxylamine to give the corresponding Weinreb amides in high yields. ... |
|
|
Nitric oxide formation from hydroxylamine by myoglobin and hydrogen peroxide.
Biochim. Biophys. Acta 1336(3) , 502-8, (1997) Hydroxylamine (HA), which is a natural product of mammalian cells, has been shown to possess vasodilatory properties in several model systems. In this study, HA and methyl-substituted hydroxylamines, N-methylhydroxylamine (NMHA) and N,N-dimethylhydroxylamine ... |
|
|
Probing the topography of the photosystem II oxygen evolving complex: PsbO is required for efficient calcium protection of the manganese cluster against dark-inhibition by an artificial reductant.
Photosynth. Res. 110(2) , 111-21, (2011) The photosystem II (PSII) manganese-stabilizing protein (PsbO) is known to be the essential PSII extrinsic subunit for stabilization and retention of the Mn and Cl(-) cofactors in the oxygen evolving complex (OEC) of PSII, but its function relative to Ca(2+) ... |
|
|
Structural studies of organoboron compounds. XVI. Preparation and crystal and molecular structures of 4, 4-dimethyl-2, 5, 5-triphenyl-1, 3-dioxa-4-azonia-2-bora-5-boratacyclopentane and 4, 4, 5, 5-tetramethyl-2, 2-diphenyl-1, 3-dioxa-4-azonia-2-boratacyclopentane.
Can. J. Chem. 62(5) , 838-844, (1984)
|