| Structure | Name/CAS No. | Articles |
|---|---|---|
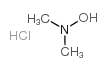 |
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE
CAS:16645-06-0 |
A R Tunoori, J M White, G I Georg
Index: Org. Lett. 2(25) , 4091-3, (2000)
Full Text: HTML
[structure] The reagent [bis(2-methoxyethyl)amino]sulfur trifluoride (Deoxo-Fluor reagent) converts carboxylic acids to the corresponding acid fluorides, which then react with N,N-dimethylhydroxylamine to give the corresponding Weinreb amides in high yields. The reaction proceeds without racemization when optically active acids are used as the starting material. This method is operationally simple and provides the products in high purity.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
N,N-DIMETHYLHYDROXYLAMINE HYDROCHLORIDE
CAS:16645-06-0 |
C2H8ClNO |
|
In vitro haematotoxic effects of three methylated hydroxylam...
1997-01-01 [Arch. Toxicol. 71(5) , 299-305, (1997)] |
|
Free radical intermediates in the oxidation of N-methylhydro...
1990-01-01 [Free Radic. Res. Commun. 8(2) , 123-31, (1990)] |
|
Two mechanisms for toxic effects of hydroxylamines in human ...
1998-09-01 [Blood Cells Mol. Dis. 24(3) , 280-95, (1998)] |
|
Nitric oxide formation from hydroxylamine by myoglobin and h...
1997-10-20 [Biochim. Biophys. Acta 1336(3) , 502-8, (1997)] |
|
Probing the topography of the photosystem II oxygen evolving...
2011-12-01 [Photosynth. Res. 110(2) , 111-21, (2011)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved