3-Maleimido-PROXYL
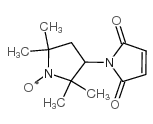
3-Maleimido-PROXYL structure
|
Common Name | 3-Maleimido-PROXYL | ||
|---|---|---|---|---|
| CAS Number | 5389-27-5 | Molecular Weight | 237.27500 | |
| Density | N/A | Boiling Point | 481.01 °C(Predicted) | |
| Molecular Formula | C12H17N2O3 | Melting Point | 111-113ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
EPR and NMR spectroscopies provide input on the coordination of Cu(I) and Ag(I) to a disordered methionine segment.
J. Biol. Inorg. Chem. 20 , 719-27, (2015) Methionine motifs are methionine-rich metal-binding segments found in many human, yeast, and bacterial proteins involved in the transportation of copper ion to other cellular pathways, and in protecting copper from oxidation. Methionine motifs are found to bi... |
|
|
A comparison between interactions of triglyceride oil and mineral oil with proteins and their ability to reduce cleanser surfactant-induced irritation.
Int. J. Cosmet. Sci. 37 , 371-8, (2015) Skin irritation in personal cleansing has been correlated with surfactant binding with stratum corneum proteins. Polar and non-polar oils are increasingly being used in cleansing formulations which contain high (10-15%) level of anionic and non-ionic surfacta... |
|
|
Interaction of transmembrane helices in ATP synthase subunit a in solution as revealed by spin label difference NMR.
Biochim. Biophys. Acta 1777(2) , 227-37, (2008) Subunit a in the membrane traversing F0 sector of Escherichia coli ATP synthase is known to fold with five transmembrane helices (TMHs) with residue 218 in TMH IV packing close to residue 248 in TMH V. In this study, we have introduced a spin label probe at C... |
|
|
The myosin catalytic domain does not rotate during the working power stroke.
Biophys. J. 69(3) , 994-9, (1995) Electron paramagnetic resonance spectroscopy of a spin probe attached to cys-707 on myosin cross-bridges was used to monitor the orientation of the myosin catalytic domain at the beginning and end of the working power stroke in active muscle. Elevated concent... |
|
|
[Modification of surface residues of lysine in immunoglobulin G using the spin marker 2,2,5,5-tetramethyl-3-maleimidopyrrolidine-1-oxyl].
Biofizika 44(5) , 806-10, (1999) Exposed lysine residues of human IgG were modified by a spin-label, 2,2,5,5-tetramethyl-3-male-imidopyrrolidine-1-oxyl at pH 9.2. Under these conditions, the degree of modification was about 10 lysine residues per protein molecule. The ESR spectrum of the spi... |
|
|
Membrane assembly of the 16-kDa proteolipid channel from Nephrops norvegicus studied by relaxation enhancements in spin-label ESR.
Biochemistry 38(43) , 14311-9, (1999) The 16-kDa proteolipid from the hepatopancreas of Nephrops norvegicus belongs to the class of channel proteins that includes the proton-translocation subunit of the vacuolar ATPases. The membranous 16-kDa protein from Nephrops was covalently spin-labeled on t... |
|
|
Rotational mobility of Ca2+-ATPase of sarcoplasmic reticulum in viscous media.
Biochim. Biophys. Acta 1326(2) , 193-200, (1997) The rotational diffusion of Ca2(+)-ATPase [Ca2+,Mg2(+)-activated ATP phosphohydrolase E.C. 3.6.1.38] was studied in native sarcoplasmic reticulum membrane by saturation transfer ESR spectroscopy after covalent labelling of intramembranous sulfhydryl groups wi... |
|
|
Electron paramagnetic resonance study of lipid and protein membrane components of erythrocytes oxidized with hydrogen peroxide.
Braz. J. Med. Biol. Res. 45(6) , 473-81, (2012) Electron paramagnetic resonance (EPR) spectroscopy of spin labels was used to monitor membrane dynamic changes in erythrocytes subjected to oxidative stress with hydrogen peroxide (H(2)O(2)). The lipid spin label, 5-doxyl stearic acid, responded to dramatic r... |
|
|
Detection of conformational changes in complex III of the respiratory chain by a maleimido spin label.
J. Bioenerg. Biomembr. 11(3-4) , 79-95, (1979) Changes in the conformation of Complex III (CoQH2-cytochrome c reductase) of the mitochondrial respiratory chain were detected upon oxidoreduction using the nitroxide spin label, 3-(maleimidomethyl)-2,2,5,5-tetramethyl-1-pyrrolidinyloxyl. EPR spectra of the s... |
|
|
Spin labelling of Bacillus anthracis endospores: a model for in vivo tracking by EPR imaging.
Free Radic. Res. 42(1) , 49-56, (2008) Anthrax is caused by the gram-negative bacterium, Bacillus anthracis. Infection by this microbe results from delivery of the endospore form of the bacillus through direct contact, either topical or inhalation. With regard to the latter route of administration... |