Rotational mobility of Ca2+-ATPase of sarcoplasmic reticulum in viscous media.
M Török, G Jakab, A Bérczi, L Dux, L I Horváth
Index: Biochim. Biophys. Acta 1326(2) , 193-200, (1997)
Full Text: HTML
Abstract
The rotational diffusion of Ca2(+)-ATPase [Ca2+,Mg2(+)-activated ATP phosphohydrolase E.C. 3.6.1.38] was studied in native sarcoplasmic reticulum membrane by saturation transfer ESR spectroscopy after covalent labelling of intramembranous sulfhydryl groups with nitroxyl derivative of maleimide (5-MSL) as a function of sucrose and glycerol in the suspending medium. The relative enzymatic activity of sarcoplasmic reticulum was followed by increasing the viscosity of the aqueous phase. The ATP hydrolysing activity of the enzyme decreased differently on adding sucrose and glycerol. In the case of sucrose the reciprocal of power dependence of viscosity was observed, whereas for glycerol an exponential decay law was obtained, indicating solvent-protein interaction. On increasing the viscosity of the aqueous phase by either sucrose or glycerol, no changes were observed in the intramembranous viscosity as measured using intercalated spin-labelled stearic acid (16-SASL). The effective rotational correlation time of the protein was measured, as a mobility parameter, using saturation transfer ESR spectroscopy and found to be increased linearly with the viscosity of the sucrose containing medium and for the extramembranous size a height of 6.8 nm was obtained, indicating that approx. 82% of the volume of Ca2(+)-ATPase protein is external to the sarcoplasmic reticulum. The addition of glycerol probably promoted protein-protein interaction, as indicated by the larger changes in rotational diffusion and non-linear viscosity dependence.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
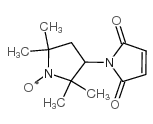 |
3-Maleimido-PROXYL
CAS:5389-27-5 |
C12H17N2O3 |
|
EPR and NMR spectroscopies provide input on the coordination...
2015-06-01 [J. Biol. Inorg. Chem. 20 , 719-27, (2015)] |
|
A comparison between interactions of triglyceride oil and mi...
2015-08-01 [Int. J. Cosmet. Sci. 37 , 371-8, (2015)] |
|
Interaction of transmembrane helices in ATP synthase subunit...
2008-02-01 [Biochim. Biophys. Acta 1777(2) , 227-37, (2008)] |
|
The myosin catalytic domain does not rotate during the worki...
1995-09-01 [Biophys. J. 69(3) , 994-9, (1995)] |
|
[Modification of surface residues of lysine in immunoglobuli...
1999-01-01 [Biofizika 44(5) , 806-10, (1999)] |