Avobenzone
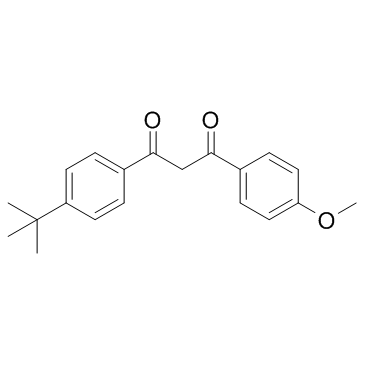
Avobenzone structure
|
Common Name | Avobenzone | ||
|---|---|---|---|---|
| CAS Number | 70356-09-1 | Molecular Weight | 310.387 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 463.6±35.0 °C at 760 mmHg | |
| Molecular Formula | C20H22O3 | Melting Point | 81-84 °C | |
| MSDS | Chinese USA | Flash Point | 203.1±26.0 °C | |
| Symbol |

GHS09 |
Signal Word | Warning | |
|
Atmospheric pressure gas chromatography-time-of-flight-mass spectrometry (APGC-ToF-MS) for the determination of regulated and emerging contaminants in aqueous samples after stir bar sorptive extraction (SBSE).
Anal. Chim. Acta 851 , 1-13, (2014) This work presents the development, optimization and validation of a multi-residue method for the simultaneous determination of 102 contaminants, including fragrances, UV filters, repellents, endocrine disruptors, biocides, polycyclic aromatic hydrocarbons (P... |
|
|
Drug-excipient compatibility studies in binary mixtures of avobenzone.
J. Cosmet. Sci. 64(5) , 317-28, (2013) During preformulation studies of cosmetic/pharmaceutical products, thermal analysis techniques are very useful to detect physical or chemical incompatibilities between the active and the excipients of interest that might interfere with safety and/or efficacy ... |
|
|
Chemical allergens stimulate human epidermal keratinocytes to produce lymphangiogenic vascular endothelial growth factor.
Toxicol. Appl. Pharmacol. 283(2) , 147-55, (2015) Allergic contact dermatitis (ACD) is a cell-mediated immune response that involves skin sensitization in response to contact with various allergens. Angiogenesis and lymphangiogenesis both play roles in the allergic sensitization process. Epidermal keratinocy... |
|
|
The risk of hydroquinone and sunscreen over-absorption via photodamaged skin is not greater in senescent skin as compared to young skin: nude mouse as an animal model.
Int. J. Pharm. 471(1-2) , 135-45, (2014) Intrinsic aging and photoaging modify skin structure and components, which subsequently change percutaneous absorption of topically applied permeants. The purpose of this study was to systematically evaluate drug/sunscreen permeation via young and senescent s... |
|
|
In vitro assessments of UVA protection by popular sunscreens available in the United States.
J. Am. Acad. Dermatol. 59(6) , 934-42, (2008) The importance of adequate ultraviolet A (UVA) protection has become apparent with improved understanding of the mechanism of UVA-induced damage to tissues. Currently in the United States, there is no regulation on testing and labeling of sunscreens for UVA p... |
|
|
The evolution of sunscreen products in the United States--a 12-year cross sectional study.
Photochem. Photobiol. Sci. 12(1) , 197-202, (2013) Excessive exposure from ultraviolet (UV) radiation contributes to the development of skin cancers and photoaging. Topical sunscreen products remain one of the most widely used forms of protection for the majority of the public. The objective of this analysis ... |
|
|
Evaluation of the photostability of different UV filter combinations in a sunscreen.
Int. J. Pharm. 307(2) , 123-8, (2006) Development of photostable sunscreens is extremely important to preserve the UV protective capacity and to prevent the reactive intermediates of photounstable filter substances behaving as photo-oxidants when coming into direct contact with the skin. Thus, th... |
|
|
Photodegradation of dibenzoylmethanes: potential cause of photocontact allergy to sunscreens.
Chem. Res. Toxicol. 22(11) , 1881-92, (2009) One of the most frequently observed photoallergens today is the sunscreen agent 4-tert-butyl-4'-methoxy dibenzoylmethane (1a). The structurally similar compound, 4-isopropyldibenzoylmethane (1b), was a common cause of sunscreen allergy in the eighties and ear... |
|
|
Transient enol isomers of dibenzoylmethane and avobenzone as efficient hydrogen donors toward a nitroxide pre-fluorescent probe.
Photochem. Photobiol. 83(3) , 481-5, (2007) Dibenzoylmethane and avobenzone photochemistry involves the formation of transient enol isomers (Z and E). Conjugation of the OH group with the carbonyl group in these transient isomers reduces the OH bond energy. A fast reduction of the pre-fluorescent probe... |
|
|
Measuring sunscreen protection against solar-simulated radiation-induced structural radical damage to skin using ESR/spin trapping: development of an ex vivo test method.
Free Radic. Res. 46(3) , 265-75, (2012) The in vitro star system used for sunscreen UVA-testing is not an absolute measure of skin protection being a ratio of the total integrated UVA/UVB absorption. The in vivo persistent-pigment-darkening method requires human volunteers. We investigated the use ... |