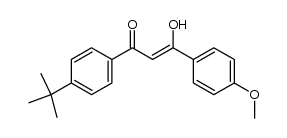
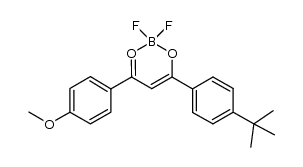
Avobenzone
Avobenzone structure
|
Common Name | Avobenzone | ||
|---|---|---|---|---|
| CAS Number | 70356-09-1 | Molecular Weight | 310.387 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 463.6±35.0 °C at 760 mmHg | |
| Molecular Formula | C20H22O3 | Melting Point | 81-84 °C | |
| MSDS | Chinese USA | Flash Point | 203.1±26.0 °C | |
| Symbol |

GHS09 |
Signal Word | Warning | |
Use of AvobenzoneAvobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative.Target: OthersAvobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative. It can degrade faster in light in combination with mineral UV absorbers like zinc oxide and titanium dioxide, though with the right coating of the mineral particles this reaction can be reduced. A manganese doped titanium dioxide may be better than undoped titanium dioxide to improve avobenzone's stability. It reacts with minerals to form colored complexes. Manufacturers of avobenzone, like DSM recommend to include a chelator to prevent this from happening. They also recommend to avoid the inclusion of iron and ferric salts, heavy metals, formaldehyde donors and PABA and PABA esters[1]. |
| Name | 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)-1,3-propanedione |
|---|---|
| Synonym | More Synonyms |
| Description | Avobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative.Target: OthersAvobenzone is an oil soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays and a dibenzoylmethane derivative. It can degrade faster in light in combination with mineral UV absorbers like zinc oxide and titanium dioxide, though with the right coating of the mineral particles this reaction can be reduced. A manganese doped titanium dioxide may be better than undoped titanium dioxide to improve avobenzone's stability. It reacts with minerals to form colored complexes. Manufacturers of avobenzone, like DSM recommend to include a chelator to prevent this from happening. They also recommend to avoid the inclusion of iron and ferric salts, heavy metals, formaldehyde donors and PABA and PABA esters[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 463.6±35.0 °C at 760 mmHg |
| Melting Point | 81-84 °C |
| Molecular Formula | C20H22O3 |
| Molecular Weight | 310.387 |
| Flash Point | 203.1±26.0 °C |
| Exact Mass | 310.156891 |
| PSA | 43.37000 |
| LogP | 4.81 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.545 |
|
~96% 
Avobenzone CAS#:70356-09-1 |
| Literature: DSM IP ASSETS B.V.; WEHRLI, Christof Patent: WO2012/84770 A1, 2012 ; Location in patent: Page/Page column 8-9 ; |
|
~93% 
Avobenzone CAS#:70356-09-1 |
| Literature: DSM IP ASSETS B.V.; WEHRLI, Christof Patent: WO2012/84770 A1, 2012 ; Location in patent: Page/Page column 10-11 ; |
|
~% 
Avobenzone CAS#:70356-09-1 |
| Literature: US2013/58879 A1, ; Paragraph 0194 ; |
|
~% 
Avobenzone CAS#:70356-09-1 |
| Literature: Journal de Chimie Physique et de Physico-Chimie Biologique, , vol. 95, # 2 p. 388 - 394 |
|
~% 
Avobenzone CAS#:70356-09-1 |
| Literature: Journal of Photochemistry and Photobiology A: Chemistry, , vol. 209, # 2-3 p. 153 - 157 |
|
~% 
Avobenzone CAS#:70356-09-1 |
| Literature: RSC Advances, , vol. 3, # 43 p. 19785 - 19788 |
| HS Code | 2909499000 |
|---|---|
| Summary | 2909499000. ether-alcohols and their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Atmospheric pressure gas chromatography-time-of-flight-mass spectrometry (APGC-ToF-MS) for the determination of regulated and emerging contaminants in aqueous samples after stir bar sorptive extraction (SBSE).
Anal. Chim. Acta 851 , 1-13, (2014) This work presents the development, optimization and validation of a multi-residue method for the simultaneous determination of 102 contaminants, including fragrances, UV filters, repellents, endocrin... |
|
|
Drug-excipient compatibility studies in binary mixtures of avobenzone.
J. Cosmet. Sci. 64(5) , 317-28, (2013) During preformulation studies of cosmetic/pharmaceutical products, thermal analysis techniques are very useful to detect physical or chemical incompatibilities between the active and the excipients of... |
|
|
Chemical allergens stimulate human epidermal keratinocytes to produce lymphangiogenic vascular endothelial growth factor.
Toxicol. Appl. Pharmacol. 283(2) , 147-55, (2015) Allergic contact dermatitis (ACD) is a cell-mediated immune response that involves skin sensitization in response to contact with various allergens. Angiogenesis and lymphangiogenesis both play roles ... |
| 1-[4-(1,1-Dimethylethyl)phenyl]-3-(4-methoxyphenyl)-1,3-propanedione |
| MFCD00210252 |
| 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione |
| 1,3-Propanedione, 1-[4-(1,1-dimethylethyl)phenyl]-3-(4-methoxyphenyl)- |
| 4-tert-Butyl-4'-methoxydibenzoylmethane |
| Avobenzone |
| 1-(4-Methoxyphenyl)-3-[4-(2-methyl-2-propanyl)phenyl]-1,3-propanedione |
| 1X1&1&R DV1VR DO1 |
| EINECS 274-581-6 |
| Avobenzone (USP) |