Acetanisole
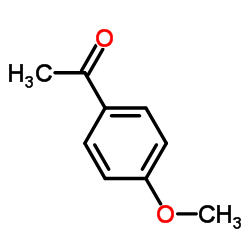
Acetanisole structure
|
Common Name | Acetanisole | ||
|---|---|---|---|---|
| CAS Number | 100-06-1 | Molecular Weight | 150.174 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 256.4±13.0 °C at 760 mmHg | |
| Molecular Formula | C9H10O2 | Melting Point | 36-38 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 113.2±13.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 4-methoxyacetophenone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 256.4±13.0 °C at 760 mmHg |
| Melting Point | 36-38 °C(lit.) |
| Molecular Formula | C9H10O2 |
| Molecular Weight | 150.174 |
| Flash Point | 113.2±13.4 °C |
| Exact Mass | 150.068085 |
| PSA | 26.30000 |
| LogP | 1.74 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.504 |
| InChIKey | NTPLXRHDUXRPNE-UHFFFAOYSA-N |
| SMILES | COc1ccc(C(C)=O)cc1 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/38 |
| Safety Phrases | S37-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AM9240000 |
| HS Code | 2914509090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Synthesis and physiological activity of thiophenes and furans with 3- and 4-methoxyacetophenone derivatives.
J. Oleo Sci. 57(2) , 107-13, (2008) The synthesis and physiological activity of thiophenes and furans with methoxyacetophenone derivatives were examined. 3-Methoxyacetophenone (1) and 4-methoxyacetophenone (2) were converted, respective... |
|
|
Electronic effects of para-substitution on acetophenones in the reaction of rat liver 3alpha-hydroxysteroid dehydrogenase.
Bioorg. Med. Chem. 16 , 1084-9, (2008) Stereoselective reductive metabolism of various p-substituted acetophenone derivatives was studied using isolated rat liver 3alpha-hydroxysteroid dehydrogenase (3alpha-HSD). Kinetic experiments were p... |
|
|
1-(1-Arylethylidene)thiosemicarbazide derivatives: a new class of tyrosinase inhibitors.
Bioorg. Med. Chem. 16 , 1096-102, (2008) A series of 1-(1-arylethylidene)thiosemicarbazide compounds and their analogues were synthesized and characterized by 1H NMR, MS. Their tyrosinase inhibitory activities were investigated by an assay b... |
| Methyl 4-methoxyphenyl ketone |
| 4-Acetoanisole |
| 4’-Methoxyacetophenone |
| 1-(4-Methoxyphenyl)ethanone |
| 4‘-Methoxyacetophenone |
| Acetophenone, 4'-methoxy- |
| EINECS 202-815-9 |
| p-Acetylanisole |
| para-Methoxyacetophenone |
| p-Methoxyacetophenone |
| Acetanisole |
| Acetophenone, p-methoxy- |
| Ethanone, 1-(4-methoxyphenyl)- |
| MFCD00008745 |
| Methyl p-methoxyphenyl ketone |
| 1VR DO1 |
| 4-Acetylanisole |
| 4-Methoxyacetophenone |
| 4'-Methoxyacetophenone |
 CAS#:64-19-7
CAS#:64-19-7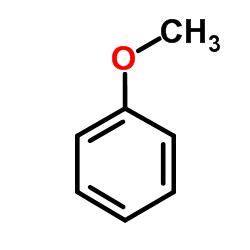 CAS#:100-66-3
CAS#:100-66-3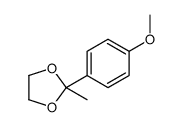 CAS#:36881-00-2
CAS#:36881-00-2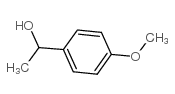 CAS#:3319-15-1
CAS#:3319-15-1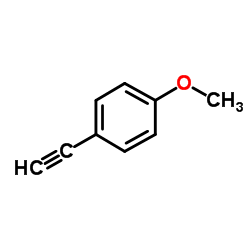 CAS#:768-60-5
CAS#:768-60-5 CAS#:108-24-7
CAS#:108-24-7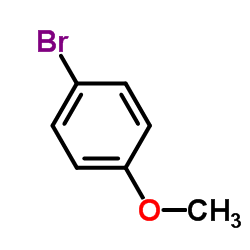 CAS#:104-92-7
CAS#:104-92-7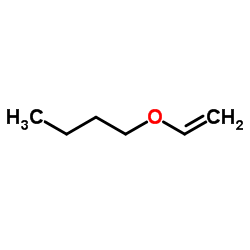 CAS#:111-34-2
CAS#:111-34-2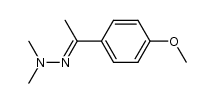 CAS#:5757-98-2
CAS#:5757-98-2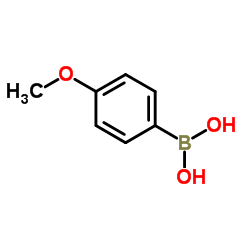 CAS#:5720-07-0
CAS#:5720-07-0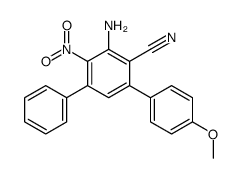 CAS#:1119522-98-3
CAS#:1119522-98-3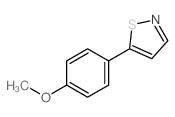 CAS#:10514-28-0
CAS#:10514-28-0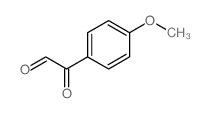 CAS#:1076-95-5
CAS#:1076-95-5 CAS#:105686-91-7
CAS#:105686-91-7 CAS#:108664-74-0
CAS#:108664-74-0 CAS#:111302-55-7
CAS#:111302-55-7![1-[2-(4-methoxybenzoyl)prop-2-enyl]pyridin-2-one structure](https://image.chemsrc.com/caspic/118/104941-13-1.png) CAS#:104941-13-1
CAS#:104941-13-1 CAS#:108779-09-5
CAS#:108779-09-5![3-[3-(4-METHOXY-PHENYL)-1-PHENYL-1H-PYRAZOL-4-YL]-ACRYLIC ACID structure](https://image.chemsrc.com/caspic/206/108446-75-9.png) CAS#:108446-75-9
CAS#:108446-75-9 CAS#:53732-47-1
CAS#:53732-47-1
