| Structure | Name/CAS No. | Articles |
|---|---|---|
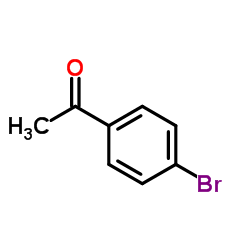 |
1-(4-Bromophenyl)ethanone
CAS:99-90-1 |
|
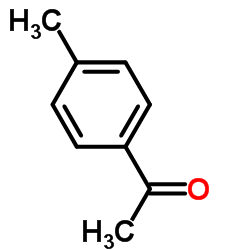 |
4'-Methylacetophenone
CAS:122-00-9 |
|
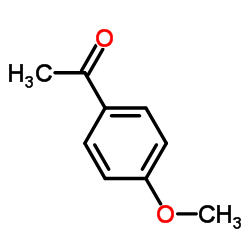 |
Acetanisole
CAS:100-06-1 |
|
 |
Acetophenone
CAS:98-86-2 |
|
 |
3alpha-hydroxysteroid dehydrogenase
CAS:9028-56-2 |
|
 |
P-CYANOACETOPHENONE
CAS:1443-80-7 |