| Structure | Name/CAS No. | Articles |
|---|---|---|
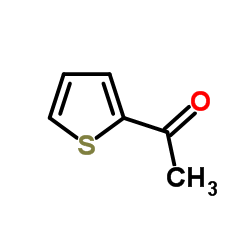 |
2-Acetylthiophene
CAS:88-15-3 |
|
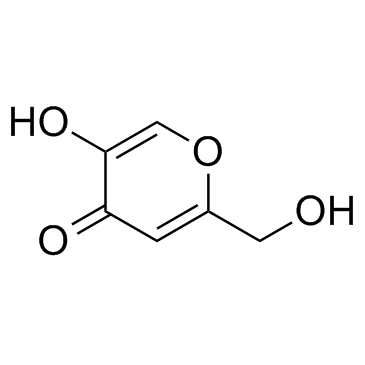 |
kojic acid
CAS:501-30-4 |
|
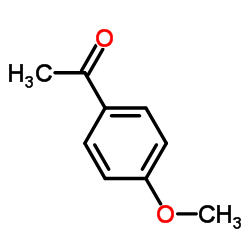 |
Acetanisole
CAS:100-06-1 |
|
 |
Acetophenone
CAS:98-86-2 |
|
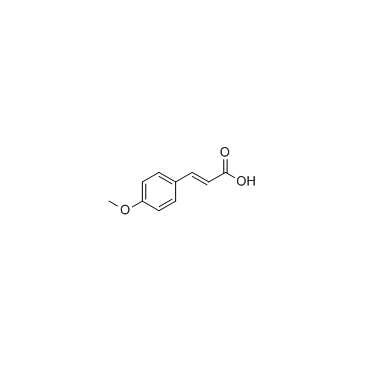 |
4-Methoxycinnamic acid
CAS:830-09-1 |
|
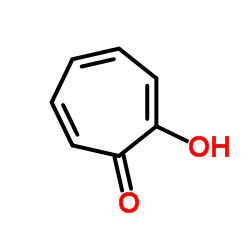 |
Tropolone
CAS:533-75-5 |
|
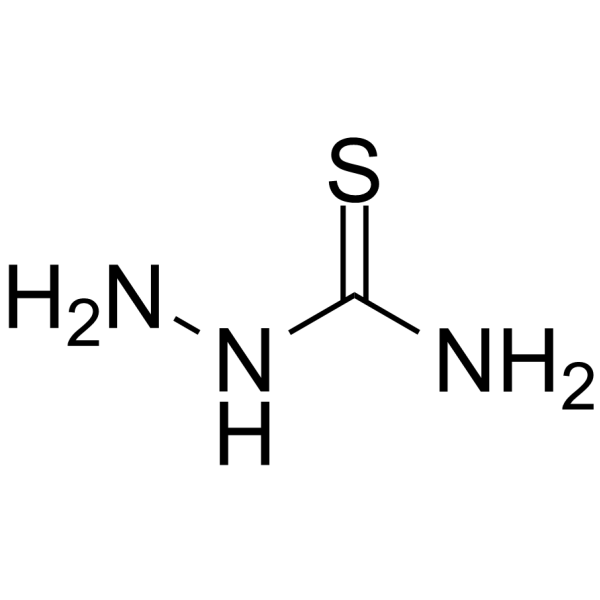 |
Thiosemicarbazide
CAS:79-19-6 |