4-Bromoanisole
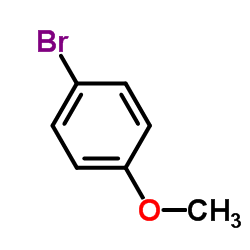
4-Bromoanisole structure
|
Common Name | 4-Bromoanisole | ||
|---|---|---|---|---|
| CAS Number | 104-92-7 | Molecular Weight | 187.034 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 223.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C7H7BrO | Melting Point | 9-10 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 94.4±0.0 °C | |
| Name | 4-bromoanisole |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 223.0±0.0 °C at 760 mmHg |
| Melting Point | 9-10 °C(lit.) |
| Molecular Formula | C7H7BrO |
| Molecular Weight | 187.034 |
| Flash Point | 94.4±0.0 °C |
| Exact Mass | 185.968018 |
| PSA | 9.23000 |
| LogP | 3.16 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.539 |
| InChIKey | QJPJQTDYNZXKQF-UHFFFAOYSA-N |
| SMILES | COc1ccc(Br)cc1 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | immiscible |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | S23-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | BZ8501000 |
| Hazard Class | 6.1 |
| HS Code | 29093038 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2909309090 |
|---|---|
| Summary | 2909309090 other aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Contributions of BrCl, Br2, BrOCl, Br2O, and HOBr to regiospecific bromination rates of anisole and bromoanisoles in aqueous solution.
Environ. Sci. Technol. 49(8) , 4937-45, (2015) When bromide-containing waters are chlorinated, conventional wisdom typically assumes HOBr is the only active brominating agent. Several additional and often-overlooked brominating agents (including B... |
|
|
Hypobromous acid, a powerful endogenous electrophile: Experimental and theoretical studies.
J. Inorg. Biochem. 146 , 61-8, (2015) Hypobromous acid (HOBr) is an inorganic acid produced by the oxidation of the bromide anion (Br(-)). The blood plasma level of Br(-) is more than 1,000-fold lower than that of chloride anion (Cl(-)). ... |
|
|
Pd-catalyzed carbonylative α-arylation of aryl bromides: scope and mechanistic studies.
Chemistry 19(52) , 17926-38, (2013) Reaction conditions for the three-component synthesis of aryl 1,3-diketones are reported applying the palladium-catalyzed carbonylative α-arylation of ketones with aryl bromides. The optimal condition... |
| Benzene, 1-bromo-4-methoxy- |
| Of BroMoanisole |
| 1-Bromo-4-methoxybenzene |
| Anisole, p-bromo- |
| p-Methoxyphenyl Bromide |
| p-anisylbromide |
| p-bromoanisol |
| Anisyl bromide |
| 4-methoxyphenyl bromide |
| 4-Bromoanisole |
| 1-bromo-4-methoxy-benzene |
| 4-BROMO ANISOL |
| 4-methoxy-1-bromobenzene |
| p-Bromanisole |
| anisole, 4-bromo- |
| EINECS 203-252-1 |
| 4-Bromophenyl methyl ether |
| p-bromoanisole |
| MFCD00000097 |
 CAS#:104-94-9
CAS#:104-94-9 CAS#:100-66-3
CAS#:100-66-3 CAS#:5720-07-0
CAS#:5720-07-0 CAS#:106-41-2
CAS#:106-41-2 CAS#:77-78-1
CAS#:77-78-1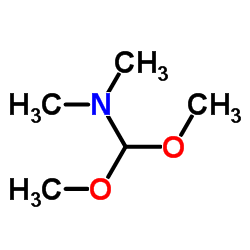 CAS#:4637-24-5
CAS#:4637-24-5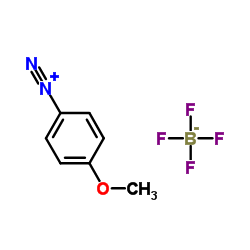 CAS#:459-64-3
CAS#:459-64-3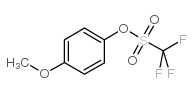 CAS#:66107-29-7
CAS#:66107-29-7 CAS#:74-88-4
CAS#:74-88-4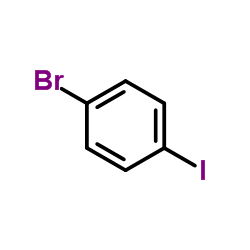 CAS#:589-87-7
CAS#:589-87-7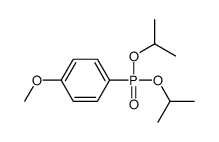 CAS#:106052-22-6
CAS#:106052-22-6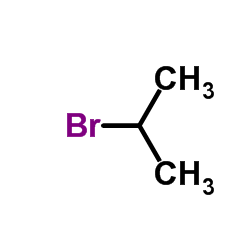 CAS#:75-26-3
CAS#:75-26-3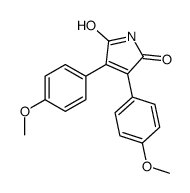 CAS#:108774-82-9
CAS#:108774-82-9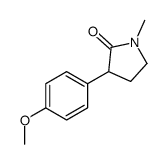 CAS#:107770-12-7
CAS#:107770-12-7 CAS#:77801-57-1
CAS#:77801-57-1 CAS#:105903-05-7
CAS#:105903-05-7![4-[2-bis(4-hydroxyphenyl)phosphanylethyl-(4-hydroxyphenyl)phosphanyl]phenol structure](https://image.chemsrc.com/caspic/411/110391-34-9.png) CAS#:110391-34-9
CAS#:110391-34-9 CAS#:108030-81-5
CAS#:108030-81-5![5-(4-METHOXYPHENYL)-[2,2']BITHIOPHENYL structure](https://image.chemsrc.com/caspic/042/106925-79-5.png) CAS#:106925-79-5
CAS#:106925-79-5![3-methoxy-6-[2-(3,4,5-trimethoxyphenyl)ethyl]benzene-1,2-diol structure](https://image.chemsrc.com/caspic/357/109971-64-4.png) CAS#:109971-64-4
CAS#:109971-64-4
