Etiracetam
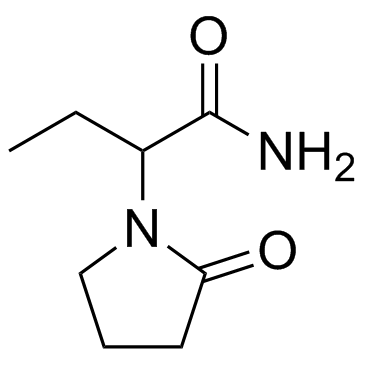
Etiracetam structure
|
Common Name | Etiracetam | ||
|---|---|---|---|---|
| CAS Number | 33996-58-6 | Molecular Weight | 170.209 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 395.9±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H14N2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 193.2±23.2 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
|
Inhibition of excitatory synaptic transmission in hippocampal neurons by levetiracetam involves Zn²⁺-dependent GABA type A receptor-mediated presynaptic modulation.
J. Pharmacol. Exp. Ther. 348(2) , 246-59, (2014) Levetiracetam (LEV) is an antiepileptic drug with a unique but as yet not fully resolved mechanism of action. Therefore, by use of a simplified rat-isolated nerve-bouton preparation, we have investigated how LEV modulates glutamatergic transmission from mossy... |
|
|
Efficacy and safety of pregabalin versus levetiracetam as adjunctive therapy in patients with partial seizures: a randomized, double-blind, noninferiority trial.
Epilepsia 55(7) , 1048-57, (2014) To assess the comparative efficacy and safety of pregabalin and levetiracetam for the reduction of seizure frequency in patients with partial seizures.This was a randomized, double-blind, flexible-dose, parallel-group noninferiority study of pregabalin and le... |
|
|
The established status epilepticus trial 2013.
Epilepsia 54 Suppl 6 , 89-92, (2013) Benzodiazepine-refractory status epilepticus (established status epilepticus, ESE) is a relatively common emergency condition with several widely used treatments. There are no controlled, randomized, blinded clinical trials to compare the efficacy and tolerab... |
|
|
The safety and tolerability of different intravenous administrations of levetiracetam, bolus versus infusion, in intensive care unit patients.
Clin. EEG Neurosci. 45(2) , 89-91, (2014) This study reviews our experience with the safety and tolerability of levetiracetam (LVM) with different methods of intravenous administration in intensive care unit (ICU) patients. We used retrospective chart review to identify 33 ICU patients who received i... |
|
|
Effects of new antiepileptic drugs on circulatory markers for vascular risk in patients with newly diagnosed epilepsy.
Epilepsia 54(10) , e146-9, (2013) Although it is well documented that long-term therapy with older antiepileptic drugs (AEDs) leads to an increase in risk for atherosclerosis, there has been only limited information regarding the vascular risk in patients who are treated with new AEDs. We the... |
|
|
Results of phase II levetiracetam trial following acute head injury in children at risk for posttraumatic epilepsy.
Epilepsia 54(9) , e135-7, (2013) Posttraumatic seizures develop in up to 20% of children following severe traumatic brain injury (TBI). Children ages 6-17 years with one or more risk factors for the development of posttraumatic epilepsy, including presence of intracranial hemorrhage, depress... |
|
|
Valproic acid, but not levetiracetam, selectively decreases HDAC7 and HDAC2 expression in human ovarian cancer cells.
Toxicol. Lett. 224(2) , 225-32, (2014) Histone deacetylases (HDACs) are often overexpressed in cancer cells, leading to altered expression and activity of numerous proteins involved in carcinogenesis. Recent evidence suggests that expression of class I HDACs is increased in ovarian carcinomas and ... |
|
|
[Efficacy and psychiatric adverse events as short-term adjunctive levetiracetam for epileptic children with refractory convulsive seizures].
No To Hattatsu. 45(6) , 463-4, (2013)
|
|
|
[Epileptic encephalopathy associated with forced normalization after administration of levetiracetam].
No To Hattatsu. 45(5) , 375-8, (2013) Here we report a case of a 10-year-old female with unclassified epileptic encephalopathy who showed forced normalization after administration of levetiracetam (LEV). She initially presented with intractable tonic and myoclonic seizures that were observed abou... |
|
|
[Efficacy and safety of levetiracetam as adjunctive therapy in Japanese children with uncontrolled partial-onset seizures: multicenter and open-label study (N01223), short term evaluation].
Brain Nerve 65(9) , 1083-92, (2013) A multicenter, open-label, single-armed study (N01223) was conducted to evaluate efficacy and safety of levetiracetam (LEV) as an add-on therapy in Japanese pediatric patients with uncontrolled partial-onset seizures (POS). A total of 73 children aged 4-15 ye... |