SAH
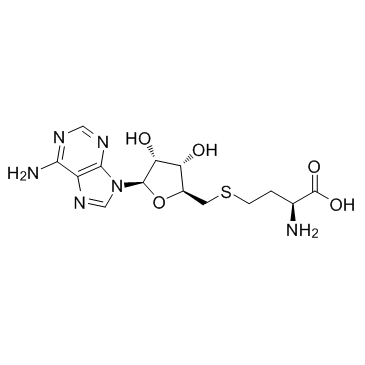
SAH structure
|
Common Name | SAH | ||
|---|---|---|---|---|
| CAS Number | 979-92-0 | Molecular Weight | 384.411 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 787.5±70.0 °C at 760 mmHg | |
| Molecular Formula | C14H20N6O5S | Melting Point | N/A | |
| MSDS | USA | Flash Point | 430.0±35.7 °C | |
|
EZH2 modulates angiogenesis in vitro and in a mouse model of limb ischemia.
Mol. Ther. 23(1) , 32-42, (2015) Epigenetic mechanisms may regulate the expression of pro-angiogenic genes, thus affecting reparative angiogenesis in ischemic limbs. The enhancer of zest homolog-2 (EZH2) induces thtrimethylation of lysine 27 on histone H3 (H3K27me3), which represses gene tra... |
|
|
Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.
Nature 457(7231) , 910-4, (2009) Multiple, complex molecular events characterize cancer development and progression. Deciphering the molecular networks that distinguish organ-confined disease from metastatic disease may lead to the identification of critical biomarkers for cancer invasion an... |
|
|
Characterization of RimO, a new member of the methylthiotransferase subclass of the radical SAM superfamily.
Biochemistry 48(42) , 10162-74, (2009) RimO, encoded by the yliG gene in Escherichia coli, has been recently identified in vivo as the enzyme responsible for the attachment of a methylthio group on the beta-carbon of Asp88 of the small ribosomal protein S12 [Anton, B. P., Saleh, L., Benner, J. S.,... |
|
|
Metabolomics of adherent mammalian cells by capillary electrophoresis-mass spectrometry: HT-29 cells as case study.
J. Pharm. Biomed. Anal. 110 , 83-92, (2015) In this work, the optimization of an effective protocol for cell metabolomics is described with special emphasis in the sample preparation and subsequent analysis of intracellular metabolites from adherent mammalian cells by capillary electrophoresis-mass spe... |
|
|
Reciprocal regulation of C-Maf tyrosine phosphorylation by Tec and Ptpn22.
PLoS ONE 10 , e0127617, (2015) C-Maf plays an important role in regulating cytokine production in TH cells. Its transactivation of IL-4 is optimized by phosphorylation at Tyr21, Tyr92, and Tyr131. However, the molecular mechanism regulating its tyrosine phosphorylation remains unknown. In ... |
|
|
Dual Targeting of the Protein Methyltransferase PrmA Contributes to Both Chloroplastic and Mitochondrial Ribosomal Protein L11 Methylation in Arabidopsis.
Plant Cell Physiol. 56 , 1697-710, (2015) Methylation of ribosomal proteins has long been described in prokaryotes and eukaryotes, but our knowledge about the enzymes responsible for these modifications in plants is scarce. The bacterial protein methyltransferase PrmA catalyzes the trimethylation of ... |
|
|
Exometabolom analysis of breast cancer cell lines: Metabolic signature.
Sci. Rep. 5 , 13374, (2015) Cancer cells show characteristic effects on cellular turnover and DNA/RNA modifications leading to elevated levels of excreted modified nucleosides. We investigated the molecular signature of different subtypes of breast cancer cell lines and the breast epith... |
|
|
Alpha-tocopherol in the ventricular cerebrospinal fluid of Parkinson's disease patients: dose-response study and correlations with plasma levels.
Neurology 47(4) , 1037-42, (1996) To determine if ventricular cerebrospinal fluid (vCSF) alpha-tocopherol levels in Parkinson's disease (PD) patients can be increased by oral alpha-tocopherol supplementation and whether vCSF levels are linearly related to plasma alpha-tocopherol levels.In spi... |
|
|
Markers of dopamine metabolism in Parkinson's disease. The Parkinson Study Group.
Neurology 42(11) , 2111-7, (1992) We used two analytic methods (a multichannel coulometric electrode array with high-performance liquid chromatography, and gas chromatography-mass spectrophotometry) to measure CSF dopamine (DA) and its metabolites in mildly affected, unmedicated subjects with... |
|
|
The formation of 3-hydroxy-4-methoxyphenylalanine and 3-hydroxy-4-methoxyphenethylamine in plasma during L-DOPA therapy in patients with Parkinson's disease.
Chem. Pharm. Bull. 32(8) , 3320-2, (1984)
|