SAH
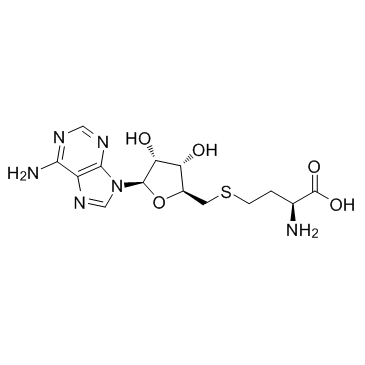
SAH structure
|
Common Name | SAH | ||
|---|---|---|---|---|
| CAS Number | 979-92-0 | Molecular Weight | 384.411 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 787.5±70.0 °C at 760 mmHg | |
| Molecular Formula | C14H20N6O5S | Melting Point | N/A | |
| MSDS | USA | Flash Point | 430.0±35.7 °C | |
Use of SAHSAH is an amino acid derivative and a modulartor in several metabolic pathways. It is an intermediate in the synthesis of cysteine and adenosine. |
| Name | S-adenosyl-L-homocysteine |
|---|---|
| Synonym | More Synonyms |
| Description | SAH is an amino acid derivative and a modulartor in several metabolic pathways. It is an intermediate in the synthesis of cysteine and adenosine. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 787.5±70.0 °C at 760 mmHg |
| Molecular Formula | C14H20N6O5S |
| Molecular Weight | 384.411 |
| Flash Point | 430.0±35.7 °C |
| Exact Mass | 384.121582 |
| PSA | 207.93000 |
| LogP | 0.15 |
| Appearance of Characters | crystalline |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.839 |
| Storage condition | −20°C |
| Water Solubility | 1 M HCl: soluble19.60 - 20.40mg/mL, clear to slightly hazy, colorless to faintly yellow |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
EZH2 modulates angiogenesis in vitro and in a mouse model of limb ischemia.
Mol. Ther. 23(1) , 32-42, (2015) Epigenetic mechanisms may regulate the expression of pro-angiogenic genes, thus affecting reparative angiogenesis in ischemic limbs. The enhancer of zest homolog-2 (EZH2) induces thtrimethylation of l... |
|
|
Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.
Nature 457(7231) , 910-4, (2009) Multiple, complex molecular events characterize cancer development and progression. Deciphering the molecular networks that distinguish organ-confined disease from metastatic disease may lead to the i... |
|
|
Characterization of RimO, a new member of the methylthiotransferase subclass of the radical SAM superfamily.
Biochemistry 48(42) , 10162-74, (2009) RimO, encoded by the yliG gene in Escherichia coli, has been recently identified in vivo as the enzyme responsible for the attachment of a methylthio group on the beta-carbon of Asp88 of the small rib... |
| S-(5'-Adenosyl)-L-homocysteine |
| (2S)-adenosylhomocysteine |
| Adenosylhomocysteine |
| Adenosyl-L-homocysteine |
| AdenosylhoMocysteine-13C5 |
| (2S)-2-Amino-4-({[(2S,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydro-2-furanyl]methyl}sulfanyl)butanoic acid (non-preferred name) |
| HOMOCYSTEINE,S-ADENOSYL |
| L-S-AdenosylhoMocysteine |
| s-adenosylhomocysteine |
| S-ADENOSYL-L-HOMOCYSTEINE |
| L-adenosyl-L-homocysteine |
| hpce |
| ADOHCY |
| 5'-DEOXY-S-ADENOSYL |
 CAS#:454-29-5
CAS#:454-29-5 CAS#:58-61-7
CAS#:58-61-7 CAS#:6027-13-0
CAS#:6027-13-0 CAS#:2140-03-6
CAS#:2140-03-6 CAS#:626-72-2
CAS#:626-72-2 CAS#:24514-56-5
CAS#:24514-56-5 CAS#:892-48-8
CAS#:892-48-8 CAS#:56-65-5
CAS#:56-65-5![5'-[[(3S)-3-AMINO-3-CARBOXYPROPYL]METHYLSULFONIO]-5'-DEOXY-ADENOSINE IODIDE structure](https://image.chemsrc.com/caspic/439/3493-13-8.png) CAS#:3493-13-8
CAS#:3493-13-8
