Sodamide
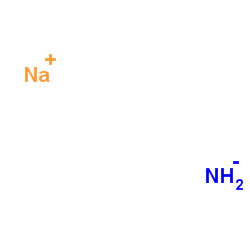
Sodamide structure
|
Common Name | Sodamide | ||
|---|---|---|---|---|
| CAS Number | 7782-92-5 | Molecular Weight | 39.012 | |
| Density | 1.4 | Boiling Point | 400 °C(lit.) | |
| Molecular Formula | H2NNa | Melting Point | 210 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 85 °F | |
| Symbol |



GHS02, GHS05, GHS09 |
Signal Word | Danger | |
|
Europium bis(dimethylsilyl)amides including mixed-valent Eu3[N(SiHMe2)2]6[μ-N(SiHMe2)2]2.
Dalton Trans. 43(46) , 17324-32, (2014) Trivalent Eu[N(SiHMe2)2]3(THF)2 can easily be synthesized by applying a routine salt metathesis protocol (EuCl3(THF)2 and 3 equiv. of Li[N(SiHMe2)2] in n-hexane) which crystallizes isotypically to its analogues of the rare-earth metal series (space group P21/... |
|
|
Isotopic studies of the ammonia decomposition reaction mediated by sodium amide.
Phys. Chem. Chem. Phys. 17 , 22999-3006, (2015) We demonstrate that the ammonia decomposition reaction catalysed by sodium amide proceeds under a different mechanism to ammonia decomposition over transition metal catalysts. Isotopic variants of ammonia and sodium amide reveal a significant kinetic isotope ... |
|
|
Stannylated allyl carbonates as versatile building blocks for the diversity oriented synthesis of allylic amines and amides.
Org. Biomol. Chem. 9 , 2743-2750, (2011) Stannylated allylic carbonates are suitable substrates for Pd-catalyzed allylic aminations. In DMF and with [allylPdCl](2) as catalyst the stannylated allyl amines formed can be directly coupled with electrophiles according to the Stille protocol, giving rise... |
|
|
M. Bakavoli, et al.,
J. Heterocycl. Chem. 48 , 149-152, (2010)
|
|
|
C. Caubere, et al.,
Tetrahedron 50 , 11903-11920, (1994)
|
|
|
K. G. Hampton, et al.,
Organic Synth. 51 , 128-132, (1971)
|