Alpha-Santonin
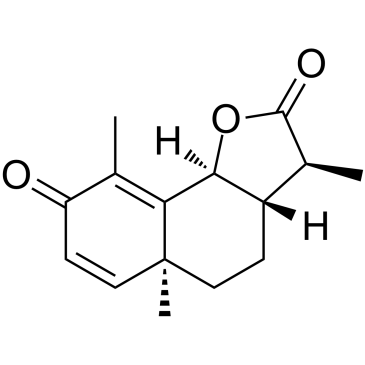
Alpha-Santonin structure
|
Common Name | Alpha-Santonin | ||
|---|---|---|---|---|
| CAS Number | 481-06-1 | Molecular Weight | 246.30200 | |
| Density | 1.18g/cm3 | Boiling Point | 423.4ºC at 760mmHg | |
| Molecular Formula | C15H18O3 | Melting Point | 172-173 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 189.7ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
The human bitter taste receptor TAS2R10 is tailored to accommodate numerous diverse ligands.
J. Neurosci. 33(1) , 201-13, (2013) Bitter taste is a basic taste modality, required to safeguard animals against consuming toxic substances. Bitter compounds are recognized by G-protein-coupled bitter taste receptors (TAS2Rs). The human TAS2R10 responds to the toxic strychnine and numerous oth... |
|
|
Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones.
Pharmacol. Biochem. Behav. 89(2) , 160-70, (2008) Here we report the prevention and reversal of cocaine-induced behaviors in planarian worms by parthenolide and two related cyclic sesquiterpene lactones (SL), costunolide and santonin. Using established protocols, we studied two cocaine-induced behavioral eff... |
|
|
Diminutive effect on T and B-cell proliferation of non-cytotoxic α-santonin derived 1,2,3-triazoles: a report.
Eur. J. Med. Chem. 60 , 365-75, (2013) α-Santonin derived new series of 1,2,3-triazoles synthesized through Azide-Alkyne Huisgen 1,3-dipolar cycloaddition reaction between substituted aryl azide and a propargylated α-desmotrosantonin were bio-evaluated for their diminutive effect on ConA induced T... |
|
|
Chemical modification of santonin into a diacetoxy acetal form confers the ability to induce differentiation of human promyelocytic leukemia cells via the down-regulation of NF-kappaB DNA binding activity.
J. Biol. Chem. 281(19) , 13117-25, (2006) Many sesquiterpene lactone compounds either induce or enhance the cell differentiation of human leukemia cells. However, we reported in a previous study that santonin, a eudesmanolide sesquiterpene lactone, exerts no effects on the differentiation of leukemia... |
|
|
Santonin-related compound 2 inhibits the nuclear translocation of NF-κB subunit p65 by targeting cysteine 38 in TNF-α-induced NF-κB signaling pathway.
Biosci. Biotechnol. Biochem. 76(12) , 2360-3, (2012) (11S)-2α-Bromo-3-oxoeudesmano-12,6α-lactone, designated santonin-related compound 2 (SRC2), only weakly affected IκBα degradation after tumor necrosis factor-α (TNF-α) stimulation, but strongly blocked the nuclear translocation of nuclear factor κB (NF-κB) su... |
|
|
Biotransformation of alpha- and 6beta-santonin by fungus and plant cell cultures.
J. Asian Nat. Prod. Res. 8(4) , 317-26, (2006) One fungus, Abisidia coerulea IFO 4011, and suspended cell cultures of one plant, Asparagus officinalis, were employed to bioconvert alpha- and 6beta-santonin. Incubation of alpha-santonin with the cell cultures of the fungus afforded two products, 11beta-hyd... |
|
|
Biotransformation of alpha-santonin by cell suspension cultures of five plants.
Biotechnol. Lett. 27(11) , 793-7, (2005) Cell suspension cultures of five plants (Catharanthus roseus, Ginkgo biloba, Platycodon grandiflorum, Taxus cuspidata, Phytolacca asinosa) were employed to bioconvert the eudesmanolide compound, alpha-santonin. Reactions occurring were hydroxylation (C-1, C-1... |
|
|
Biotransformation of tetrahydro-alpha-santonins by Absidia coerulea.
Nat. Prod. Res. 22(6) , 499-506, (2008) The fungus, Absidia coerulea was employed to bioconvert tetrahydro-alpha-santonins, 1,2,4alpha,5alpha-tetrahydro-alpha-santonin (1), and its 4-epimer (2), from which 10 products (3-12) were obtained. Furthermore, their structures were determined, based on the... |
|
|
Microbial transformations of alpha-santonin.
Z. Naturforsch., C, J. Biosci. 59(3-4) , 209-14, (2004) Fungal biotransformations of alpha-santonin (1) were conducted with Mucor plumbeus (ATCC 4740), Cunninghamella bainieri (ATCC 9244), Cunninghamella echinulata (ATCC 9245), Curvularia lunata (ATCC 12017) and Rhizopus stolonifer (ATCC 10404). Rhizopus stolonife... |
|
|
Synthesis of diacetoxy acetal derivatives of santonin and their enhancing effects on HL-60 leukemia cell differentiation.
Arch. Pharm. Res. 29(1) , 40-5, (2006) Several diacetoxy acetal analogues have been synthesized from santonin and assessed for their ability of inducing or enhancing the differentiation of human HL-60 leukemia cells. The compounds themselves had little effect on HL-60 cell differentiation. However... |