Synthesis of diacetoxy acetal derivatives of santonin and their enhancing effects on HL-60 leukemia cell differentiation.
Seung Hyun Kim, Sun Young Chung, Tae Sung Kim, Bo Gil Choi
Index: Arch. Pharm. Res. 29(1) , 40-5, (2006)
Full Text: HTML
Abstract
Several diacetoxy acetal analogues have been synthesized from santonin and assessed for their ability of inducing or enhancing the differentiation of human HL-60 leukemia cells. The compounds themselves had little effect on HL-60 cell differentiation. However, three analogues, 2a, 3a, and 5b, synergistically enhanced 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3]-induced HL-60 cell differentiation when combined with 5 nM of dihydroxyvitamin D3 [1,25-(OH)2D3], a well-known differentiation inducer. Especially, the compound 5b profoundly enhanced the 1,25-(OH)2D3]-induced HL-60 cell differentiation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
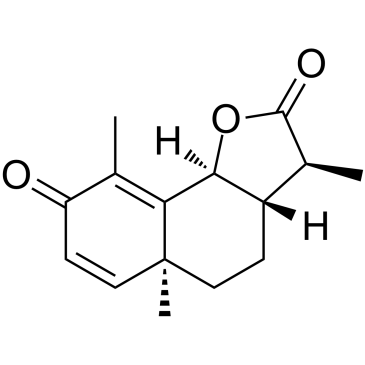 |
Alpha-Santonin
CAS:481-06-1 |
C15H18O3 |
|
The human bitter taste receptor TAS2R10 is tailored to accom...
2013-01-02 [J. Neurosci. 33(1) , 201-13, (2013)] |
|
Reversal of cocaine-induced planarian behavior by parthenoli...
2008-04-01 [Pharmacol. Biochem. Behav. 89(2) , 160-70, (2008)] |
|
Diminutive effect on T and B-cell proliferation of non-cytot...
2013-02-01 [Eur. J. Med. Chem. 60 , 365-75, (2013)] |
|
Chemical modification of santonin into a diacetoxy acetal fo...
2006-05-12 [J. Biol. Chem. 281(19) , 13117-25, (2006)] |
|
Santonin-related compound 2 inhibits the nuclear translocati...
2012-01-01 [Biosci. Biotechnol. Biochem. 76(12) , 2360-3, (2012)] |