cefaclor
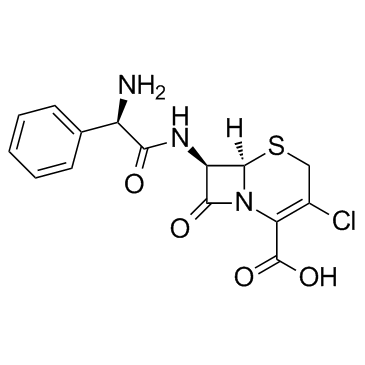
cefaclor structure
|
Common Name | cefaclor | ||
|---|---|---|---|---|
| CAS Number | 53994-73-3 | Molecular Weight | 367.807 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 713.4±60.0 °C at 760 mmHg | |
| Molecular Formula | C15H14ClN3O4S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 385.2±32.9 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Hologram QSAR model for the prediction of human oral bioavailability.
Bioorg. Med. Chem. 15 , 7738-45, (2007) A drug intended for use in humans should have an ideal balance of pharmacokinetics and safety, as well as potency and selectivity. Unfavorable pharmacokinetics can negatively affect the clinical development of many otherwise promising drug candidates. A varie... |
|
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared and assayed 33 coumarin derivatives, and the theoretical pr... |
|
|
Exploring the potential impact of an expanded genetic code on protein function.
Proc. Natl. Acad. Sci. U. S. A. 112 , 6961-6, (2015) With few exceptions, all living organisms encode the same 20 canonical amino acids; however, it remains an open question whether organisms with additional amino acids beyond the common 20 might have an evolutionary advantage. Here, we begin to test that notio... |
|
|
Membrane transporters in drug development.
Nat. Rev. Drug Discov. 9 , 215-236, (2010) Membrane transporters can be major determinants of the pharmacokinetic, safety and efficacy profiles of drugs. This presents several key questions for drug development, including which transporters are clinically important in drug absorption and disposition, ... |
|
|
Determination of cefaclor by UPLC-MS-MS for a Chinese pharmacokinetic study.
J. Chromatogr. Sci. 52(7) , 636-40, (2014) A novel method has been developed for the determination of cefaclor in human plasma by ultra-performance liquid chromatography combined with tandem mass spectrometry (UPLC-MS-MS). The plasma was treated by a single step of protein precipitation with acetonitr... |
|
|
Biocompatible hydrodispersible magnetite nanoparticles used as antibiotic drug carriers.
Rom. J. Morphol. Embryol. 56 , 365-70, (2015) Here we report a newly synthesized vectorizing nanosystem, based on hydrodispersible magnetite nanoparticles (HMNPs) with an average size less than 10 nm, obtained by precipitation of Fe(II) and Fe(III) in basic solution of p-aminobenzoic acid (PABA), charact... |
|
|
New valid spectrofluorimetric method for determination of selected cephalosporins in different pharmaceutical formulations using safranin as fluorophore.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 153 , 655-60, (2015) A new validated spectrofluorimetric method has been developed for the determination of some cephalosporins namely; cefepime, cefaclor, cefadroxil, cefpodoxime and cefexime. The method was based on the reaction of these drugs with safranin in slightly alkaline... |