1-AZIDO-4-IODOBENZENE SOLUTION
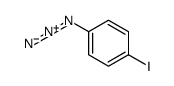
1-AZIDO-4-IODOBENZENE SOLUTION structure
|
Common Name | 1-AZIDO-4-IODOBENZENE SOLUTION | ||
|---|---|---|---|---|
| CAS Number | 53694-87-4 | Molecular Weight | 245.02100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H4IN3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | -33℃ | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Danger | |
|
Modification of ovine opsin with the photosensitive hydrophobic probe 1-azido-4-[125I]iodobenzene. Labelling of the chromophore-attachment domain.
Biochem. J. 234(2) , 413-20, (1986) The hydrophobic photosensitive probe 1-azido-4-[125I]iodobenzene (AIB) partitioned preferentially into photoreceptor disc membranes and, upon u.v. irradiation, became covalently bound to opsin and phospholipid. The labelling of both protein and phospholipid w... |
|
|
Identification of the sites in opsin modified by photoactivated azido[125I]iodobenzene.
Biochem. J. 236(2) , 389-95, (1986) Opsin labelled with photoactivated 1-azido-4-[125I]iodobenzene was proteolysed in situ with Staphylococcus aureus V8 proteinase to yield two radioactive membrane-bound fragments. These were separated, cleaved with CNBr and the resultant peptides sequenced in ... |
|
|
Influence of diet on the susceptibility of Xenopus laevis tadpoles to thiopentone and a membrane probe.
Br. J. Anaesth. 53(6) , 577-84, (1981) Xenopus laevis tadpoles were produced from wild-caught, laboratory-reared toads. Separate sets were fed on diets of (1) liver powder; (2) nettle powder; (3) aminosol and Intralipid. The tadpoles were reared for 3 weeks at 22 degrees C and then three groups of... |
|
|
The isolation of human-erythrocyte band-3 polypeptide labelled with a photosensitive hydrophobic probe.
Biochem. J. 187(3) , 719-25, (1980) To investigate the intramembranous domains of the major band-3 polypeptide, human erythrocyte membranes were labelled with 1-azido-4-[125I]iodobenzene. The anion-exchange protein has been isolated by a new procedure that decreases possible contamination by ot... |
|
|
Labeling of hydrophobic polypeptides from the chick lens membrane.
Exp. Eye Res. 35(5) , 535-40, (1982)
|