Cercosporamide
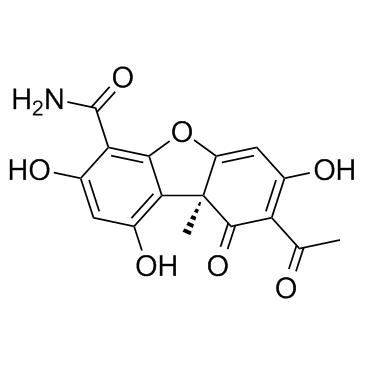
Cercosporamide structure
|
Common Name | Cercosporamide | ||
|---|---|---|---|---|
| CAS Number | 131436-22-1 | Molecular Weight | 331.277 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 582.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H13NO7 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 306.1±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens.
Appl. Microbiol. Biotechnol. 93(3) , 1231-9, (2012) Through bioassay-guided fractionation, the EtOAc extract of a culture broth of the endophytic fungus Phoma species ZJWCF006 in Arisaema erubescens afforded a new α-tetralone derivative, (3S)-3,6,7-trihydroxy-α-tetralone (1), together with cercosporamide (2), ... |
|
|
Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors.
Blood 121(18) , 3675-81, (2013) Mnk kinases regulate the phosphorylation and activation of the eukaryotic initiation factor 4E (eIF4E), a protein that plays key roles in the initiation of messenger RNA translation and whose activity is critical for various cellular functions. eIF4E is dereg... |
|
|
Cloning and characterization of KNR4, a yeast gene involved in (1,3)-beta-glucan synthesis.
Mol. Cell. Biol. 14(2) , 1017-25, (1994) k9 killer toxin from Hansenula mrakii was used to select a number of resistant mutants from Saccharomyces cerevisiae. Preliminary biochemical and genetic studies showed that some of them acquired structural defects in the cell wall. One of these mutants, the ... |
|
|
Fermentative production of self-toxic fungal secondary metabolites.
J. Ind. Microbiol. Biotechnol. 37(4) , 335-40, (2010) Fungi are well known for their vast diversity of secondary metabolites that include many life-saving drugs and highly toxic mycotoxins. In general, fungal cultures producing such metabolites are immune to their toxic effects. However, some are known to produc... |
|
|
Synthesis and biological evaluation of novel (-)-Cercosporamide derivatives as potent selective PPARγ modulators.
Eur. J. Med. Chem. 54 , 522-33, (2012) Selective peroxisome proliferator-activated receptor gamma (PPARγ) modulators are expected to be a novel class of drugs improving plasma glucose levels without PPARγ-related adverse effects. As a continuation of our studies for (-)-Cercosporamide derivatives ... |
|
|
Discovery of a novel selective PPARgamma modulator from (-)-Cercosporamide derivatives.
Bioorg. Med. Chem. Lett. 20(7) , 2095-8, (2010) In an investigation of (-)-Cercosporamide derivatives with a plasma glucose-lowering effect, we found that N-benzylcarboxamide derivative 4 was a partial agonist of PPARgamma. A SAR study of the substituents on carboxamide nitrogen afforded the N-(1-naphthyl)... |
|
|
Substituents at the naphthalene C3 position of (-)-Cercosporamide derivatives significantly affect the maximal efficacy as PPARγ partial agonists.
Bioorg. Med. Chem. Lett. 22(3) , 1348-51, (2012) Peroxisome proliferator-activated receptor gamma (PPARγ) is a potential drug target for treating type 2 diabetes. The selective PPARγ modulators (SPPARMs), which partially activate the PPARγ transcriptional activity, are considered to improve the plasma gluco... |
|
|
Pharmacology and in vitro profiling of a novel peroxisome proliferator-activated receptor γ ligand, Cerco-A.
Biol. Pharm. Bull. 34(7) , 1094-104, (2011) Peroxisome proliferator-activated receptor γ (PPARγ; NR1C3) is known as a key regulator of adipocytogenesis and the molecular target of thiazolidinediones (TZDs), also known as antidiabetic agents. Despite the clinical benefits of TZDs, their use is often ass... |