Substituents at the naphthalene C3 position of (-)-Cercosporamide derivatives significantly affect the maximal efficacy as PPARγ partial agonists.
Akihiro Furukawa, Tsuyoshi Arita, Takehiro Fukuzaki, Susumu Satoh, Makoto Mori, Takeshi Honda, Yumi Matsui, Kenji Wakabayashi, Shinko Hayashi, Kazushi Araki, Jun Ohsumi
Index: Bioorg. Med. Chem. Lett. 22(3) , 1348-51, (2012)
Full Text: HTML
Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) is a potential drug target for treating type 2 diabetes. The selective PPARγ modulators (SPPARMs), which partially activate the PPARγ transcriptional activity, are considered to improve the plasma glucose level with attenuated PPARγ related adverse effects. However, the relationships between desired pharmacological profiles and ligand specific PPARγ transcriptional profiles have been unclear. And there is also little knowledge of how to control ligand specific PPARγ transcriptional profiles. Herein, we present synthesis of novel derivatives containing substituent at naphthalene C3 position of compound 1. The novel derivatives showed various maximal efficacies as PPARγ partial agonist.Copyright © 2011 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
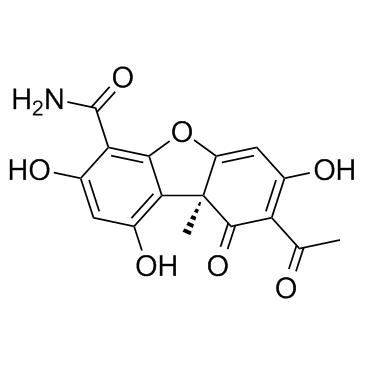 |
Cercosporamide
CAS:131436-22-1 |
C16H13NO7 |
|
Bioactive metabolites from Phoma species, an endophytic fung...
2012-02-01 [Appl. Microbiol. Biotechnol. 93(3) , 1231-9, (2012)] |
|
Inhibition of Mnk kinase activity by cercosporamide and supp...
2013-05-02 [Blood 121(18) , 3675-81, (2013)] |
|
Cloning and characterization of KNR4, a yeast gene involved ...
1994-02-01 [Mol. Cell. Biol. 14(2) , 1017-25, (1994)] |
|
Fermentative production of self-toxic fungal secondary metab...
2010-04-01 [J. Ind. Microbiol. Biotechnol. 37(4) , 335-40, (2010)] |
|
Synthesis and biological evaluation of novel (-)-Cercosporam...
2012-08-01 [Eur. J. Med. Chem. 54 , 522-33, (2012)] |