6-Methoxyindole
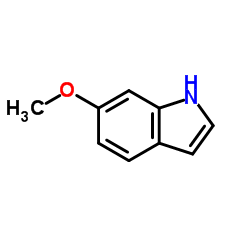
6-Methoxyindole structure
|
Common Name | 6-Methoxyindole | ||
|---|---|---|---|---|
| CAS Number | 3189-13-7 | Molecular Weight | 147.174 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 297.8±13.0 °C at 760 mmHg | |
| Molecular Formula | C9H9NO | Melting Point | 90-92 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 109.2±10.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators.
J. Med. Chem. 54 , 5320, (2011) Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance. IDO inhibition is thus an active area of research in drug development. Recently, our group ... |
|
|
Discovery of a novel class of PPARdelta partial agonists.
Bioorg. Med. Chem. Lett. 18 , 5018, (2008) Anthranilic acid GW9371 was identified as a novel class of PPARdelta partial agonist through high-throughput screening. The design and synthesis of SAR analogues is described. GSK1115 and GSK7227 show potent partial agonism of the PPARdelta target genes CPT1a... |
|
|
Synthesis and evaluation of 3-aroylindoles as anticancer agents: metabolite approach.
J. Med. Chem. 52 , 4941, (2009) BPR0L075 (2) is a potential anticancer drug candidate designed from Combretastatin A-4 (1) based on the bioisosterism principle. Metabolites of 2, proposed from in vitro human microsome studies, were synthesized, leading to the identification of metabolite-de... |
|
|
Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity.
Bioorg. Med. Chem. Lett. 20 , 5229, (2010) A simple and highly efficient method has been developed for the synthesis of 3,3-diindolyl oxyindoles by the reaction of indoles with isatin or 5-fluoro isatin using a catalytic amount (5 mol%) of FeCl(3) at room temperature in a short reaction time in high y... |
|
|
Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid.
Braz. J. Med. Biol. Res. 38(11) , 1575-83, (2005) Hypochlorous acid (HOCl) released by activated leukocytes has been implicated in the tissue damage that characterizes chronic inflammatory diseases. In this investigation, 14 indole derivatives, including metabolites such as melatonin, tryptophan and indole-3... |
|
|
Identification of potent ITK inhibitors through focused compound library design including structural information
Bioorg. Med. Chem. Lett. 20 , 6998, (2010) A series of novel compound libraries inhibiting interleukin-2 inducible T cell kinase (ITK) were designed, synthesized and evaluated. In the first design cycle two library scaffolds were identified showing low micromolar inhibition of ITK. Further iterative d... |
|
|
Synthesis and evaluation of heteroaryl-ketone derivatives as a novel class of VEGFR-2 inhibitors.
Bioorg. Med. Chem. Lett. 18 , 4344, (2008) We have discovered novel inhibitors of VEGFR-2 kinase with low nanomolar potency in both enzymatic and cell-based assays. Active series are heteroaryl-ketone compounds containing a central aromatic ring with either an indazolyl or indolyl keto group in the or... |
|
|
Fluorescence of protonated excited-state forms of 5-hydroxytryptamine (serotonin) and related indoles.
Proc. Natl. Acad. Sci. U. S. A. 60(2) , 598-605, (1968)
|
|
|
Meanwell, N., A.; et al.
Bioorg. Med. Chem. Lett. 20 , 1460, (2010)
|