Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity.
Ahmed Kamal, Y V V Srikanth, M Naseer A Khan, Thokhir Basha Shaik, Md Ashraf
Index: Bioorg. Med. Chem. Lett. 20 , 5229, (2010)
Full Text: HTML
Abstract
A simple and highly efficient method has been developed for the synthesis of 3,3-diindolyl oxyindoles by the reaction of indoles with isatin or 5-fluoro isatin using a catalytic amount (5 mol%) of FeCl(3) at room temperature in a short reaction time in high yields. All these compounds were evaluated against a panel of five human cancer lines and most of them showed potent cytotoxicity. Compound 4b showed IC(50) of 4.7 and 5 microM against SK-N-SH and DU-145 cell lines, respectively, whereas 4c, 4d, 4f and 4k showed IC(50) of 2.2, 1.2, 3.6 and 3.6 microM, respectively, against DU-145 cell line. Interestingly, some of the compounds are selectively potent in prostate cancer (DU-145) with IC(50) values of 1.2-19.6 microM.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
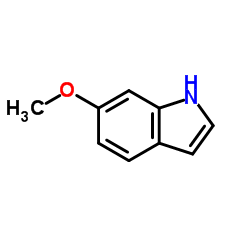 |
6-Methoxyindole
CAS:3189-13-7 |
C9H9NO |
|
Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)e...
2011-08-11 [J. Med. Chem. 54 , 5320, (2011)] |
|
Discovery of a novel class of PPARdelta partial agonists.
2008-09-15 [Bioorg. Med. Chem. Lett. 18 , 5018, (2008)] |
|
Synthesis and evaluation of 3-aroylindoles as anticancer age...
2009-08-13 [J. Med. Chem. 52 , 4941, (2009)] |
|
Indole ring oxidation by activated leukocytes prevents the p...
2005-11-01 [Braz. J. Med. Biol. Res. 38(11) , 1575-83, (2005)] |
|
Identification of potent ITK inhibitors through focused comp...
2010-01-01 [Bioorg. Med. Chem. Lett. 20 , 6998, (2010)] |