Dihydrokavain
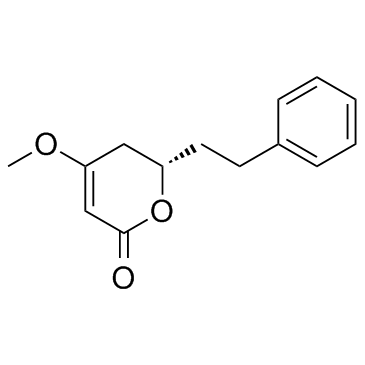
Dihydrokavain structure
|
Common Name | Dihydrokavain | ||
|---|---|---|---|---|
| CAS Number | 587-63-3 | Molecular Weight | 232.275 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 413.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C14H16O3 | Melting Point | 56-60ºC | |
| MSDS | Chinese USA | Flash Point | 175.6±23.3 °C | |
|
Interaction of various Piper methysticum cultivars with CNS receptors in vitro.
Planta Med. 67(4) , 306-11, (2001) Methanolic leaf and root extracts of the Hawaiian kava (Piper methysticum Forst.) cultivars, Mahakea, Nene, Purple Moi and PNG, were tested on binding affinities to CNS receptors including GABAA (GABA and benzodiazepine binding site), dopamine D2, opioid (mu ... |
|
|
Determination of six kavalactones in dietary supplements and selected functional foods containing Piper methysticum by isocratic liquid chromatography with internal standard.
J. AOAC Int. 88(1) , 16-25, (2005) Kava (Piper methysticum) dietary products have been sold worldwide for treatment of nervous anxiety, tension, and restlessness. Recent reports showed potential association of kava usage and liver injuries. This study was conducted to develop simple and reliab... |
|
|
Glial dysfunction in the mouse habenula causes depressive-like behaviors and sleep disturbance.
J. Neurosci. 34(49) , 16273-85, (2014) The lateral habenula (LHb) regulates the activity of monoaminergic neurons in the brainstem. This area has recently attracted a surge of interest in psychiatry because studies have reported the pathological activation of the habenula in patients with major de... |
|
|
Identification and characterization of kava-derived compounds mediating TNF-alpha suppression.
Chem. Biol. Drug Des. 74(2) , 121-8, (2009) There is a substantial unmet need for new classes of drugs that block TNF-alpha-mediated inflammation, and particularly for small molecule agents that can be taken orally. We have screened a library of natural products against an assay measuring TNF-alpha sec... |
|
|
Efficient enantioselective hetero-Diels-Alder reaction of Brassard's diene with aliphatic aldehydes: a one-step synthesis of (R)-(+)-kavain and (S)-(+)-dihydrokavain.
Org. Lett. 10(6) , 1311-4, (2008) An efficient catalytic asymmetric hetero-Diels-Alder reaction of Brassard's diene with aliphatic aldehydes was reported. The catalyst, which was generated from (R)-BINOL, Ti(i-PrO)4, and 4-picolyl chloride hydrochloride, promoted the reaction smoothly to affo... |
|
|
Extracts and kavalactones of Piper methysticum G. Forst (kava-kava) inhibit P-glycoprotein in vitro.
Drug Metab. Dispos. 33(11) , 1580-3, (2005) Root extracts from kava-kava (Piper methysticum G. Forst) are clinically used for the treatment of anxiety and restlessness. Due to reported cases of liver toxicity, kava-kava extracts were withdrawn from the market in several countries in 2002. Because the e... |
|
|
Inhibition of cytochrome P450 3A4 by extracts and kavalactones of Piper methysticum (Kava-Kava).
Planta Med. 68(12) , 1055-8, (2002) Inhibitors of cytochrome P450 3A4 (CYP3A4) were identified in crude extracts from the rhizomes of Piper methysticum G. Forst. (Kava-Kava) using bioassay-guided fractionation. After preliminary purification of an ethyl acetate extract with solid phase extracti... |
|
|
Anxiolytic effects of kava extract and kavalactones in the chick social separation-stress paradigm.
Psychopharmacology 155(1) , 86-90, (2001) Piper methysticum extract (kava kava) possesses numerous therapeutic properties, but it is unknown which of its principle constituents (kavalactones) subserve such effects.This experiment sought to characterize the putative anxiolytic properties of P. methyst... |
|
|
Cyclodextrins as carriers for kavalactones in aqueous media: spectroscopic characterization of (S)-7,8-dihydrokavain and beta-cyclodextrin inclusion complex.
J. Pharm. Biomed. Anal. 52(4) , 479-83, (2010) Kavalactones represent the active constituents of kava-kava (Piper methysticum G. Forster), endowed with sedative and anaesthetic properties. Kavalactones are polar constituents, but poorly soluble in water with a low bioavailability. In this study, the forma... |
|
|
Kavalactones and dihydrokavain modulate GABAergic activity in a rat gastric-brainstem preparation.
Planta Med. 68(12) , 1092-6, (2002) Using an in vitro neonatal rat gastric-brainstem preparation, the activity of majority neurons recorded in the nucleus tractus solitarius (NTS) of the brainstem were significantly inhibited by GABA A receptor agonist, muscimol (30 microM), and this inhibition... |