N4-Benzoylcytosine
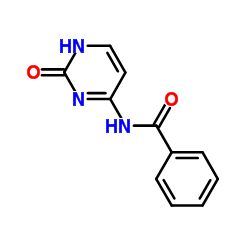
N4-Benzoylcytosine structure
|
Common Name | N4-Benzoylcytosine | ||
|---|---|---|---|---|
| CAS Number | 26661-13-2 | Molecular Weight | 215.208 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C11H9N3O2 | Melting Point | >300 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis and biological evaluation of 3'-C-ethynyl and 3'-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides.
Med. Chem. 8(3) , 320-9, (2012) A novel series of 3'-C-ethynyl and 3'-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides has been designed and synthesized. Reaction of 3-keto glucoside 1 with ethynyl magnesium bromide gave the desired precursor 3-C-ethynyl-1,2:5,6-di-O-iso... |
|
|
Nucleosides and nucleotides. 232. Synthesis of 2'-C-methyl-4'-thiocytidine: unexpected anomerization of the 2'-keto-4'-thionucleoside precursor.
Nucleosides Nucleotides Nucleic Acids 24(10-12) , 1789-800, (2005) The synthesis of 2'-C-methyl-4'-thiocytidine (16) is described. Since the 2'-keto-4'-thiocytidine derivative 2beta unexpectedly isomerized to 2alpha and the methylation of 2beta proceeded predominantly from the less hindered alpha-face to give 7, the desired ... |
|
|
Synthesis and anti-HIV and anti-HBV activities of 2'-fluoro-2', 3'-unsaturated L-nucleosides.
J. Med. Chem. 42(7) , 1320-8, (1999) The synthesis of L-nucleoside analogues containing 2'-vinylic fluoride was accomplished by direct condensation method, and their anti-HIV and anti-HBV activities were evaluated in vitro. The key intermediate 8, the sugar moiety of our target compounds, was pr... |
|
|
Antimicrobial activity of amide, N4-benzoylcytosine. Jagessar RC and Gomathinayagam S.
J. Pharm. Clin. Sci. 1 , 12-19, (2011)
|