N4-Benzoylcytosine
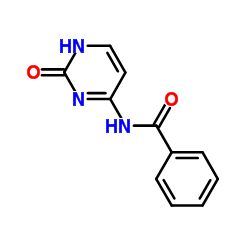
N4-Benzoylcytosine structure
|
Common Name | N4-Benzoylcytosine | ||
|---|---|---|---|---|
| CAS Number | 26661-13-2 | Molecular Weight | 215.208 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C11H9N3O2 | Melting Point | >300 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | N-(2-oxo-1H-pyrimidin-6-yl)benzamide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | >300 °C (dec.)(lit.) |
| Molecular Formula | C11H9N3O2 |
| Molecular Weight | 215.208 |
| Exact Mass | 215.069473 |
| PSA | 74.85000 |
| LogP | 0.42 |
| Index of Refraction | 1.652 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H332-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R20/22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~88% 
N4-Benzoylcytosine CAS#:26661-13-2 |
| Literature: NANYANG TECHONOLOGICAL UNIVERSITY Patent: US2011/245458 A1, 2011 ; |
|
~72% 
N4-Benzoylcytosine CAS#:26661-13-2 |
| Literature: ISIS Pharmaceuticals, Inc. Patent: US6828427 B1, 2004 ; |
|
~90% 
N4-Benzoylcytosine CAS#:26661-13-2 |
| Literature: Holy, Antonin; Rosenberg, Ivan; Dvorakova, Hana Collection of Czechoslovak Chemical Communications, 1989 , vol. 54, # 8 p. 2190 - 2210 |
|
~% 
N4-Benzoylcytosine CAS#:26661-13-2 |
| Literature: Kawaguchi; Ishikawa; Seki; Juni; Fukushima; Nakano Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 9 p. 2547 - 2549 |
| Precursor 5 | |
|---|---|
| DownStream 9 | |
|
Synthesis and biological evaluation of 3'-C-ethynyl and 3'-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides.
Med. Chem. 8(3) , 320-9, (2012) A novel series of 3'-C-ethynyl and 3'-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides has been designed and synthesized. Reaction of 3-keto glucoside 1 with ethynyl magnesium brom... |
|
|
Nucleosides and nucleotides. 232. Synthesis of 2'-C-methyl-4'-thiocytidine: unexpected anomerization of the 2'-keto-4'-thionucleoside precursor.
Nucleosides Nucleotides Nucleic Acids 24(10-12) , 1789-800, (2005) The synthesis of 2'-C-methyl-4'-thiocytidine (16) is described. Since the 2'-keto-4'-thiocytidine derivative 2beta unexpectedly isomerized to 2alpha and the methylation of 2beta proceeded predominantl... |
|
|
Synthesis and anti-HIV and anti-HBV activities of 2'-fluoro-2', 3'-unsaturated L-nucleosides.
J. Med. Chem. 42(7) , 1320-8, (1999) The synthesis of L-nucleoside analogues containing 2'-vinylic fluoride was accomplished by direct condensation method, and their anti-HIV and anti-HBV activities were evaluated in vitro. The key inter... |
| N-(2-oxohydropyrimidin-4-yl)benzamide |
| N4-Benzoylcytosine |
| N6-benzoylcytosine |
| N3-benzoylcytosine |
| N-(2-Oxo-1,2-dihydro-4-pyrimidinyl)benzamide |
| N-(2-Oxo-1,2-dihydropyrimidin-4-yl)benzamide |
| MFCD00239434 |
| N-(1,2-dihydro-2-oxopyrimidin-4-yl)-benzamide |
| N4-Benzoyl Cytosine |
| N-benzoylcytosine |
| Benzamide, N-(1,2-dihydro-2-oxo-4-pyrimidinyl)- |
| N-(2-Oxo-2,3-dihydropyrimidin-4-yl)benzamide |
| N4-Bz-cytosine |


 CAS#:23624-64-8
CAS#:23624-64-8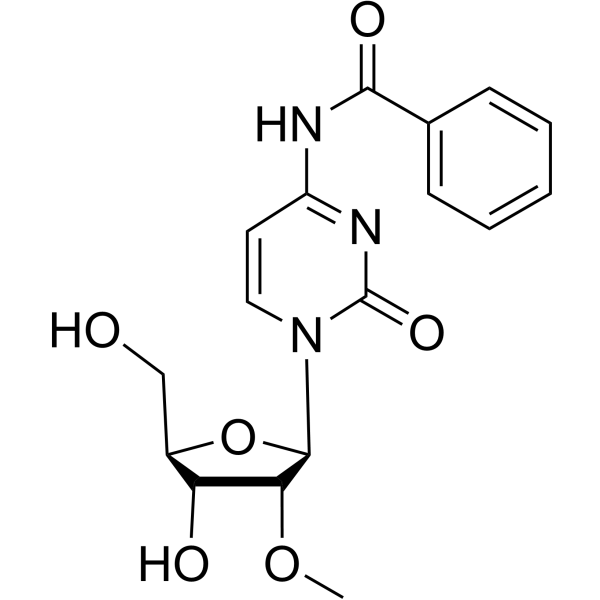 CAS#:52571-45-6
CAS#:52571-45-6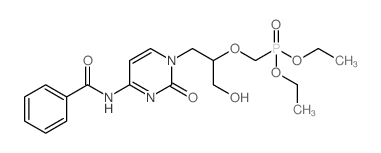 CAS#:132336-36-8
CAS#:132336-36-8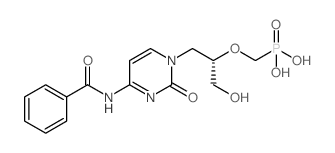 CAS#:132336-37-9
CAS#:132336-37-9![N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)thiolan-2-yl]-2-oxopyrimidin-4-yl]benzamide structure](https://image.chemsrc.com/caspic/150/159981-07-4.png) CAS#:159981-07-4
CAS#:159981-07-4![Benzamide, N-[1,2-dihydro-2-oxo-1-(2-propenyl)-4-pyrimidinyl]- (9CI) structure](https://image.chemsrc.com/caspic/406/648881-65-6.png) CAS#:648881-65-6
CAS#:648881-65-6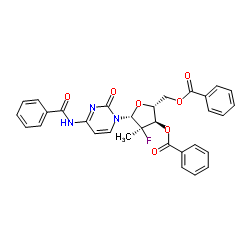 CAS#:817204-32-3
CAS#:817204-32-3 CAS#:85743-99-3
CAS#:85743-99-3 CAS#:64339-87-3
CAS#:64339-87-3
