Ammonium sulfide
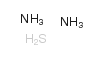
Ammonium sulfide structure
|
Common Name | Ammonium sulfide | ||
|---|---|---|---|---|
| CAS Number | 12135-76-1 | Molecular Weight | 68.14190 | |
| Density | 1 g/mL at 25 °C | Boiling Point | 40 °C | |
| Molecular Formula | H8N2S | Melting Point | -18ºC | |
| MSDS | Chinese USA | Flash Point | 90 °F | |
| Symbol |



GHS02, GHS05, GHS09 |
Signal Word | Danger | |
|
Characterization of volatile compounds from the reaction of 3-hydroxy-2-butanone and ammonium sulfide model system.
J. Agric. Food Chem. 47(1) , 245-8, (1999) The reactions between 3-hydroxy-2-butanone and ammoniun sulfide at 25, 50, 75, 100, 125, and 150 degrees C were studied. Four well-known flavor compounds, 2,4,5-trimethyloxazole, 2,4, 5-trimethyl-3-oxazoline, 2,4,5-trimethylthiazole, and 2,4, 5-trimethyl-3-th... |
|
|
A cataluminescence gas sensor for ammonium sulfide based on Fe(3)O(4)-carbon nanotubes composite.
Luminescence 25(4) , 294-9, (2010) In the present work, Fe(3)O(4)-carbon nanotubes (CNTs) composite was explored as a sensing material candidate for ammonium sulfide. Intense chemiluminescence emission can be observed during the catalytic oxidation of ammonium sulfide on the surface of Fe(3)O(... |
|
|
Visualization of iron in cultured macrophages: a cytochemical light and electron microscopic study using autometallography.
Free Radic. Biol. Med. 15(1) , 1-11, (1993) The objective of this study was to develop a sensitive cytochemical method for the visualization of iron, both at light microscopical (LM) and at electron microscopical (EM) levels, in glutaraldehyde-fixed cultured cells with reasonable morphological preserva... |
|
|
Critical period of carbon tetrachloride-induced pregnancy loss in Fischer-344 rats, with insights into the detection of resorption sites by ammonium sulfide staining.
Teratology 56(4) , 252-61, (1997) Several low-molecular weight halocarbons have been shown to cause full-litter resorption (FLR), i.e., pregnancy loss, in Fischer-344 rats treated during organogenesis. To determine periods of gestation sensitive to acute exposure, a single dose of 150 mg carb... |
|
|
The influence of odorants on respiratory patterns in sleep.
Chem. Senses 35(1) , 31-40, (2010) To assess the feasibility of using odors as a potential mechanism for treating sleep apnea, we set out to test the hypothesis that odorants delivered during sleep would modify respiratory patterns without inducing arousal or wake in healthy sleepers. We used ... |
|
|
A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography.
Clin. Chim. Acta 293(1-2) , 115-25, (2000) Hydrogen sulphide is produced in the human large intestine by the bacterial reduction of dietary inorganic sulphate and sulphite and by fermentation of sulphur amino acids. Sulphide may damage the colonic epithelium and has been implicated in the pathogenesis... |
|
|
Generation and maintenance of synchrony in Saccharomyces cerevisiae continuous culture.
Exp. Cell Res. 287(1) , 10-5, (2003) Cultures of Saccharomyces cerevisiae grown continuously produce an autonomous oscillation in many metabolic outputs. The most conveniently measured variable, i.e., dissolved oxygen concentration, oscillates with a period of 40-55 min. Previously we have ident... |
|
|
[Photographic models in the study and valuation of the silver reactions in histology. II. Optical density variation in enlarging exposure scale under the action of the ammonium sulphide on silver (author's transl)].
Mikroskopie 36(3-4) , 117-27, (1980)
|
|
|
The relationship between the surface composition and electrical properties of corrosion films formed on carbon steel in alkaline sour medium: an XPS and EIS study.
J. Phys. Chem. B 110(29) , 14398-405, (2006) This work studies the evolution of 1018 carbon steel surfaces during 3-15 day immersion in alkaline sour medium 0.1 M (NH4)2S and 10 ppm CN(-) as (NaCN). During this period of time, surfaces were jointly characterized by electrochemical techniques in situ (el... |
|
|
[Preparation and spectral properties of PVP-modified CdS nanorods].
Guang Pu Xue Yu Guang Pu Fen Xi 25(6) , 971-4, (2005) Polyvinylpyrrolidone (PVP)-modified CdS nanorods were prepared by a hydrothermal reaction of CdCl2 2.5H2O and (NH4)2S with 10 wt% ethylenediamine aqueous solution as solvent and 1.0 wt% PVP as additives. The obtained products were characterized by means of XR... |