2-methyl-m-phenylene diisocyanate
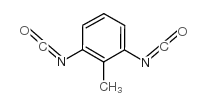
2-methyl-m-phenylene diisocyanate structure
|
Common Name | 2-methyl-m-phenylene diisocyanate | ||
|---|---|---|---|---|
| CAS Number | 91-08-7 | Molecular Weight | 174.15600 | |
| Density | 1.225 g/mL at 25 °C(lit.) | Boiling Point | 129-133 °C18 mm Hg(lit.) | |
| Molecular Formula | C9H6N2O2 | Melting Point | 13°C | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
|
Biological monitoring as a valid tool to assess occupational exposure to mixtures of 2,4-:2,6-toluene diisocyanate.
Med. Lav. 103(5) , 361-71, (2012) Despite its advantages over environmental monitoring, biological monitoring of exposure to 2,4-:2,6-toluene diisocyanate (TDI) mixtures is still underused. The present study was designed in order to evaluate the feasibility and reliability of biological monit... |
|
|
Magnetic field dependent electro-conductivity of the graphite doped magnetorheological plastomers.
Soft Matter 11 , 6893-902, (2015) In this work we reported a novel graphite doped conductive magnetorheological plastomer (GMRP) with magnetic field dependent electro-conductivity. The conductivity of the GMRPs increased by increasing the content of the graphite particles, while it decreased ... |
|
|
LC-MS determination of urinary toluenediamine in workers exposed to toluenediisocyanate.
Toxicol. Lett. 134(1-3) , 259-64, (2002) To improve the biological monitoring method for 2,6- and 2,4-toluenediisocyanate (TDI) exposure, we developed a simple and rapid method for analysis of the corresponding urinary metabolites, 2,6- and 2,4-toluenediamine (TDA) using liquid chromatograph-mass sp... |
|
|
The GSTP1 Ile105 Val polymorphism modifies the metabolism of toluene di-isocyanate.
Pharmacogenet. Genomics 20(2) , 104-11, (2010) Toluene di-isocyanate (TDI) is widely used in the production of polyurethane foams and paints. As TDI causes respiratory disease in only a fraction of exposed workers, genetic factors may play a key role in disease susceptibility. Polymorphisms in TDI metabol... |
|
|
Determination of unreacted 2,4-toluene diisocyanate (2,4TDI) and 2,6-toluene diisocyanate (2,6TDI) in foams at ultratrace level by using HPLC-CIS-MS-MS.
Analyst 128(12) , 1447-51, (2003) Isocyanates can cause occupational asthma. By using available HPLC-UVF methods, isocyanates can be quantified only at levels above 1% of the Permissible Exposure Limits (PEL). Once sensitized, workers can react to concentrations below these limits of detectio... |
|
|
Experimental study on respiratory sensitivity to inhaled toluene diisocyanate.
Arch. Toxicol. 67(6) , 373-8, (1993) Groups of guinea pigs were exposed via inhalation to toluene diisocyanate (TDI) ranging from 0.02 to 1.0 ppm for 3 h/day on 5 consecutive days. Three weeks later, guinea pigs were challenged with TDI-GSA conjugates. Evaluations were based on TDI specific anti... |
|
|
Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry.
Anal. Biochem. 414(2) , 232-8, (2011) Diisocyanates are highly reactive chemical compounds widely used in the manufacture of polyurethanes. Although diisocyanates have been identified as causative agents of allergic respiratory diseases, the specific mechanism by which these diseases occur is lar... |
|
|
An asthma model developed in the guinea pig by intranasal application of 2,4-toluene diisocyanate.
Int. Arch. Allergy Immunol. 101(1) , 95-101, (1993) Guinea pigs were sensitized with 2,4-toluene diisocyanate (TDI) by a repetitive application onto the bilateral nasal vestibules once a day for 5 consecutive days. When these animals were further treated with TDI for additional 9 weeks, a number of these anima... |
|
|
Urinary excretion of toluene diisocyanates in rats following dermal exposure.
J. Appl. Toxicol. 28(2) , 189-95, (2008) Toluene diisocyanates (TDI) are commonly used in polyurethane (PU)-related products. TDIs have been documented as the leading cause of occupational asthma. Skin exposure to TDI in the workplace is common. However, no studies in the literature have investigate... |
|
|
Quantitative determination of hexamethylene diisocyanate (HDI), 2,4-toluene diisocyanate (2,4-TDI) and 2,6-toluene diisocyanate (2,6-TDI) monomers at ppt levels in air by alkaline adduct coordination ionspray tandem mass spectrometry.
J. Environ. Monit. 7(2) , 145-50, (2005) Occupational exposures to isocyanates can lead to occupational asthma. Once sensitized, some workers could react to isocyanate monomers at concentrations below 1% of the Permissible Exposure Limit of 5 ppb in air. Currently available methods are not sufficien... |