Naphthazarin
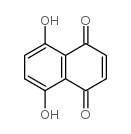
Naphthazarin structure
|
Common Name | Naphthazarin | ||
|---|---|---|---|---|
| CAS Number | 475-38-7 | Molecular Weight | 190.15200 | |
| Density | 1.592g/cm3 | Boiling Point | 498.2ºC at 760mmHg | |
| Molecular Formula | C10H6O4 | Melting Point | 220-230 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 269.2ºC | |
|
Stimulation of Suicidal Erythrocyte Death by Naphthazarin.
Basic Clin Pharmacol Toxicol. 117 , 369-74, (2016) The 1,4-naphthoquinone derivative naphthazarin may trigger apoptosis and is thus considered for the treatment of malignancy. On the other hand, naphthazarin decreases neurotoxicity. In analogy to apoptosis of nucleated cells, erythrocytes may enter eryptosis,... |
|
|
Bioactivities of simplified adociaquinone B and naphthoquinone derivatives against Cdc25B, MKP-1, and MKP-3 phosphatases.
Bioorg. Med. Chem. 17 , 2276-81, (2009) Some simplified adociaquinone B analogs and a series of 1,4-naphthoquinone derivatives were synthesized and tested against the three enzymes Cdc25B, MKP-1, and MKP-3. Cdc25B and MKP-1 in particular are enzymes overexpressed in human cancer cells, and they rep... |
|
|
Naphthazarin enhances ionizing radiation-induced cell cycle arrest and apoptosis in human breast cancer cells.
Int. J. Oncol. 46(4) , 1659-66, (2015) Naphthazarin (Naph, DHNQ, 5,8-dihydroxy-l,4-naphthoquinone) is one of the naturally available 1,4-naphthoquinone derivatives that are well-known for their anti-inflammatory, antioxidant, antibacterial and antitumor cytotoxic effects in cancer cells. Herein, w... |
|
|
Inhibitory effect of a naphthazarin derivative, S64, on heat shock factor (Hsf) activation and glutathione status following hypoxia.
Cell Biol. Toxicol. 19(5) , 273-84, (2003) The presence of hypoxic cells in solid tumors has long been considered a problem in cancer treatment. Resistance of hypoxic cells to ionizing radiation and anticancer drugs has in part been attributed to changes in altered gene expression by hypoxia. We previ... |
|
|
Effect of colloidal silver against the cytotoxicity of hydrogen peroxide and naphthazarin on primary cultured cortical astrocytes.
Int. J. Neurosci. 117(3) , 387-400, (2007) One major pathogenesis in degenerative disorders of the central nervous system (CNS), including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and ischemia, is the oxidative stress induced by reactive oxygen species (ROS). The presen... |
|
|
The microtubule depolymerizing agent naphthazarin induces both apoptosis and autophagy in A549 lung cancer cells.
Apoptosis 16(9) , 924-39, (2011) Naphthazarin (DHNQ, 5,8-dihydroxy-l,4-naphthoquinone) is a naturally available 1,4-naphthoquinone derivatives. In this study, we focused on elucidating the cytotoxic mechanism of naphthazarin in A549 non-small cell lung carcinoma cells. Naphthazarin reduced t... |
|
|
Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms.
PLoS ONE 6(7) , e21922, (2011) Hormesis occurs when a low level stress elicits adaptive beneficial responses that protect against subsequent exposure to severe stress. Recent findings suggest that mild oxidative and thermal stress can extend lifespan by hormetic mechanisms. Here we show th... |
|
|
Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation.
Phytother Res. 17(5) , 501-7, (2003) Naturally occurring gums and resins with beneficial pharmaceutical and nutraceutical properties were tested for their possible protective effect against copper-induced LDL oxidation in vitro. Chiosmastic gum (CMG) (Pistacia lentiscus var. Chia resin) was the ... |
|
|
Synthesis of (1,4)-naphthoquinono-[3,2-c]-1H-pyrazoles and their (1,4)-naphthohydroquinone derivatives as antifungal, antibacterial, and anticancer agents.
Bioorg. Med. Chem. Lett. 15 , 3288-91, (2005) A series of (1,4)-naphthoquinono [3,2-c]-1H-pyrazoles and their (1,4)-naphthohydroquinone derivatives 2-7 were synthesized and evaluated for antifungal, antibacterial, and anticancer activities. The structure-activity relationship of these compounds was studi... |
|
|
Fourier transform infrared and Raman spectra, vibrational assignment and density functional theory calculations of naphthazarin.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 60(1-2) , 111-20, (2004) FT Raman and FTIR spectra of Naphthazarin (5,8-dihydroxy-1,4-naphthoquinone) and its deuterated analogue are recorded. Comparison between the spectra obtained by two techniques, a series of density functional theory (DFT) calculations and the spectral behavio... |