Piperazine Hexahydrate
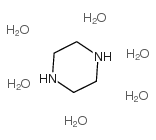
Piperazine Hexahydrate structure
|
Common Name | Piperazine Hexahydrate | ||
|---|---|---|---|---|
| CAS Number | 142-63-2 | Molecular Weight | 194.22700 | |
| Density | 1.92g/cm3 | Boiling Point | 145-156 °C(lit.) | |
| Molecular Formula | C4H22N2O6 | Melting Point | 42-44 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 190 °F | |
| Symbol |


GHS05, GHS08 |
Signal Word | Danger | |
|
Improved solvent formulations for efficient CO₂ absorption and low-temperature desorption.
ChemSusChem 5(9) , 1724-31, (2012) This experimental study describes efficient CO₂ capture by 2-amino-2-methyl-1-propanol (AMP)/piperazine (PZ) in ethylene glycol monoethyl ether (EGMEE, 2-ethoxyethanol) containing approximately 15 wt % of water. In these experiments, the solvent is continuous... |
|
|
Synthesis and anti-inflammatory effects of new piperazine and ethanolamine derivatives of H(1)-antihistaminic drugs.
Mini Rev. Med. Chem. 12(12) , 1282-92, (2012) In addition to their antihistamine effects, H1-receptor antagonists possess pharmacological properties that are not uniformly distributed among this class of drugs, such as anti-inflammatory, anti-allergic and antiplatelet activities. In this paper, Cyclizine... |
|
|
Fragment-based discovery of 8-hydroxyquinoline inhibitors of the HIV-1 integrase-lens epithelium-derived growth factor/p75 (IN-LEDGF/p75) interaction.
J. Med. Chem. 56(6) , 2311-22, (2013) On the basis of an initial molecular modeling study suggesting the favorable binding of the "privileged" fragment 8-hydroxyquinoline with HIV-1 integrase (IN) at the IN-lens epithelium-derived growth factor/p75 (LEDGF/p75) interface , we developed a set of mo... |
|
|
Homopiperazine grafted mesoporous silicas from rice husk ash for CO2 adsorption.
J. Nanosci. Nanotechnol. 14(6) , 4639-48, (2014) Chloro-functionalized mesoporous MCM-41, SBA-15, MCM-48 and KIT-6 were synthesized by co-condensation of 3-chloropropyl-trimethoxy-silane (CPTMS) and rice husk ash sodium silicate solution, which is subsequently grafted with a heterocyclic amine, homopiperazi... |
|
|
Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater.
Water Res. 46(16) , 5235-46, (2012) This study addressed the formation and properties of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed during ozonation of secondary wastewater effluent containing these fluoroquinolone antibiotics. The generation of the degradation p... |
|
|
Synthesis, characterization, antidepressant and antioxidant activity of novel piperamides bearing piperidine and piperazine analogues.
Bioorg. Med. Chem. Lett. 22(23) , 7065-70, (2012) A series of piperamide derivatives (8a-j) was synthesized with various substituted piperidine and piperazine compounds. The prepared compounds were evaluated for antibacterial activity against gram-positive and gram-negative bacteria and antifungal activity b... |
|
|
Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase.
Chem. Biol. 20(3) , 379-90, (2013) Monoacylglycerol lipase (MAGL) terminates the signaling function of the endocannabinoid, 2-arachidonoylglycerol (2-AG). During 2-AG hydrolysis, MAGL liberates arachidonic acid, feeding the principal substrate for the neuroinflammatory prostaglandins. In cance... |
|
|
Degradation of piperazine by Paracoccus sp. TOH isolated from activated sludge.
Bioresour. Technol. 130 , 536-42, (2013) Piperazine is widely used as an intermediate in the manufacture of insecticides, rubber chemicals, corrosion inhibitors, and urethane. In this study, a highly effective piperazine-degrading bacteria strain, TOH, was isolated from the acclimated activated slud... |
|
|
Metabolism, excretion, and pharmacokinetics of ((3,3-difluoropyrrolidin-1-yl)((2S,4S)-4-(4-(pyrimidin-2-yl)piperazin-1-yl)pyrrolidin-2-yl)methanone, a dipeptidyl peptidase inhibitor, in rat, dog and human.
Drug Metab. Dispos. 40(11) , 2143-61, (2012) The disposition of 3,3-difluoropyrrolidin-1-yl{(2S,4S)-4-[4-(pyrimidin-2-yl)piperazin-1-yl]pyrrolidin-2-yl}methanone (PF-00734200), a dipeptidyl peptidase IV inhibitor that progressed to phase 3 for the treatment of type 2 diabetes, was examined in rats, dogs... |
|
|
Design, structure-activity relationship and in vivo efficacy of piperazine analogues of fenarimol as inhibitors of Trypanosoma cruzi.
Bioorg. Med. Chem. 21(7) , 1756-63, (2013) A scaffold hopping exercise undertaken to expand the structural diversity of the fenarimol series of anti-Trypanosoma cruzi (T. cruzi) compounds led to preparation of simple 1-[phenyl(pyridin-3-yl)methyl]piperazinyl analogues of fenarimol which were investiga... |