Butanoic acid, ethenylester
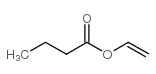
Butanoic acid, ethenylester structure
|
Common Name | Butanoic acid, ethenylester | ||
|---|---|---|---|---|
| CAS Number | 123-20-6 | Molecular Weight | 114.14200 | |
| Density | 0.899 g/mL at 20 °C | Boiling Point | 115-117°C | |
| Molecular Formula | C6H10O2 | Melting Point | -80°C | |
| MSDS | Chinese USA | Flash Point | 20°C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
|
Solid-state fermentation as a potential technique for esterase/lipase production by halophilic archaea.
Extremophiles 19 , 1121-32, (2015) Halophilic archaea are extremophiles, adapted to high-salt environments, showing a big biotechnological potential as enzyme, lipids and pigments producers. Four inert supports (perlite, vermiculite, polyurethane foam and glass fiber) were employed for solid-s... |
|
|
Enhanced productivity of electrospun polyvinyl alcohol nanofibrous mats using aqueousN,N-dimethylformamide solution and their application to lipase-immobilizing membrane-shaped catalysts
J. Biosci. Bioeng. 114(2) , 204-8, (2012) Electrospun polyvinyl alcohol (PVA) nanofibrous non-woven fabrics have been widely used for cell and enzyme immobilization. Enhancement of the productivity of the material will further enlarge the versatility of them. In this study, a mixture of water and N,N... |
|
|
Lipase-catalysed hydrolysis of short-chain substrates in solution and in emulsion: a kinetic study.
Biochim. Biophys. Acta 1534(1) , 34-44, (2001) We have studied the enzymatic hydrolysis of solutions and emulsions of vinyl propionate, vinyl butyrate and tripropionin by lipases of various origin and specificity. Kinetic studies of the hydrolysis of short-chain substrates by microbial triacylglycerol lip... |
|
|
Distinction between esterases and lipases: a kinetic study with vinyl esters and TAG.
Lipids 37(7) , 653-62, (2002) The better to characterize enzymes hydrolyzing carboxyl ester bonds (carboxyl ester hydrolases), we have compared the kinetic behavior of various lipases and esterases against solutions and emulsions of vinyl esters and TAG. Short-chain vinyl esters are hydro... |
|
|
Substrate specificity and kinetic properties of enzymes belonging to the hormone-sensitive lipase family: comparison with non-lipolytic and lipolytic carboxylesterases.
Biochim. Biophys. Acta 1738(1-3) , 29-36, (2005) We have studied the kinetics of hydrolysis of triacylglycerols, vinyl esters and p-nitrophenyl butyrate by four carboxylesterases of the HSL family, namely recombinant human hormone-sensitive lipase (HSL), EST2 from Alicyclobacillus acidocaldarius, AFEST from... |
|
|
Dynamic kinetic resolution of secondary alcohols combining enzyme-catalyzed transesterification and zeolite-catalyzed racemization.
Chemistry 13(2) , 541-7, (2007) Hydrophobic zeolite beta containing low concentrations of Zr or Al was found to be a good catalyst for the racemization of 1-phenylethanol. The formation of styrene as a side product could be minimized by reducing the metal concentration in the zeolite beta. ... |
|
|
Two-Step Enzymatic Modification of Solid-Supported Bergenin in Aqueous and Organic Media.
Tetrahedron Lett. 51(8) , 1220, (2010) The natural flavonoid bergenin was directly immobilized onto carboxylic acid functionalized controlled pore glass (carboxy-CPG) at 95% yield. Immobilized bergenin was brominated via chloroperoxidase in aqueous solution and then transesterified with vinyl buty... |
|
|
On the activity loss of hydrolases in organic solvents: II. a mechanistic study of subtilisin Carlsberg.
BMC Biotechnol. 6 , 51, (2007) Enzymes have been extensively used in organic solvents to catalyze a variety of transformations of biological and industrial significance. It has been generally accepted that in dry aprotic organic solvents, enzymes are kinetically trapped in their conformati... |
|
|
Probing a functional role of Glu87 and Trp89 in the lid of Humicola lanuginosa lipase through transesterification reactions in organic solvent.
J. Protein Chem. 14(4) , 217-24, (1995) To reveal the functional role of Glu87 and Trp89 in the lid of Humicola lanuginosa lipase, site-directed mutagenesis at Glu87 and Trp89 was carried out. The catalytic performance of wild-type and mutated lipases was studied in transesterification reactions in... |
|
|
Significant changes in the transesterification activity of free and mesoporous-immobilized Rhizopus oryzae lipase in ionic liquids.
J. Biotechnol. 145(3) , 281-3, (2010) We examined the activity of free Rhizopus oryzae lipase (ROL) and ROL immobilized on mesoporous materials in transesterification reactions in various dialkylimidazolium-cation based ionic liquids. For free ROL, the highest activity (0.39 U/mg protein) was obt... |