Two-Step Enzymatic Modification of Solid-Supported Bergenin in Aqueous and Organic Media.
Umar Akbar, Dong-Sik Shin, Elizabeth Schneider, JonathanS Dordick, DouglasS Clark
Index: Tetrahedron Lett. 51(8) , 1220, (2010)
Full Text: HTML
Abstract
The natural flavonoid bergenin was directly immobilized onto carboxylic acid functionalized controlled pore glass (carboxy-CPG) at 95% yield. Immobilized bergenin was brominated via chloroperoxidase in aqueous solution and then transesterified with vinyl butyrate in diisopropyl ether by subtilisin carslberg (SC) extracted into the organic solvent via ion pairing. Enzymatic cleavage of 7-bromo-4-butyrylbergenin from carboxy-CPG (9.6% final yield) was accomplished using lipase B (LipB) in an aqueous/organic mixture (90/10 v/v of water/acetonitrile), demonstrating the feasibility of solid phase biocatalysis of a natural product in aqueous and non-aqueous media.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
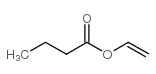 |
Butanoic acid, ethenylester
CAS:123-20-6 |
C6H10O2 |
|
Solid-state fermentation as a potential technique for estera...
2015-11-01 [Extremophiles 19 , 1121-32, (2015)] |
|
Enhanced productivity of electrospun polyvinyl alcohol nanof...
2012-08-01 [J. Biosci. Bioeng. 114(2) , 204-8, (2012)] |
|
Lipase-catalysed hydrolysis of short-chain substrates in sol...
2001-11-30 [Biochim. Biophys. Acta 1534(1) , 34-44, (2001)] |
|
Distinction between esterases and lipases: a kinetic study w...
2002-07-01 [Lipids 37(7) , 653-62, (2002)] |
|
Substrate specificity and kinetic properties of enzymes belo...
2005-12-30 [Biochim. Biophys. Acta 1738(1-3) , 29-36, (2005)] |