Ascorbyl Glucoside
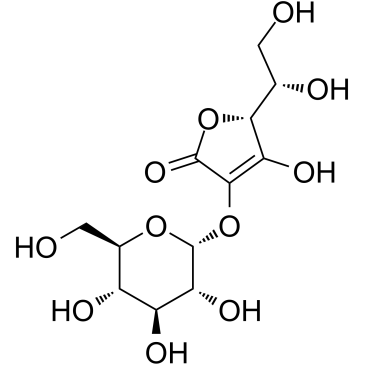
Ascorbyl Glucoside structure
|
Common Name | Ascorbyl Glucoside | ||
|---|---|---|---|---|
| CAS Number | 129499-78-1 | Molecular Weight | 338.265 | |
| Density | 1.83±0.1 g/cm3 | Boiling Point | 785.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C12H18O11 | Melting Point | 158-163℃ | |
| MSDS | Chinese USA | Flash Point | 296.5±26.4 °C | |
|
Functions, applications and production of 2-O-D-glucopyranosyl-L-ascorbic acid.
Appl. Microbiol. Biotechnol. 95(2) , 313-20, (2012) Vitamin C (VC) is an essential nutrient that cannot be synthesized by the human body. Due to its extreme instability, various VC derivatives have been developed in an attempt to improve stability while retaining the same biological activity. One of the most i... |
|
|
Systems engineering of tyrosine 195, tyrosine 260, and glutamine 265 in cyclodextrin glycosyltransferase from Paenibacillus macerans to enhance maltodextrin specificity for 2-O-(D)-glucopyranosyl-(L)-ascorbic acid synthesis.
Appl. Environ. Microbiol. 79(2) , 672-7, (2013) In this work, the site saturation mutagenesis of tyrosine 195, tyrosine 260 and glutamine 265 in the cyclodextrin glycosyltransferase (CGTase) from Paenibacillus macerans was conducted to improve the specificity of CGTase for maltodextrin, which can be used a... |
|
|
Evaluation of the antioxidative capability of commonly used antioxidants in dermocosmetics byin vivodetection of protein carbonylation in human stratum corneum
J. Photochem. Photobiol. B, Biol. 112 , 7-15, (2012) Graphical abstract |
|
|
Regioselective monoacylation of 2-O-α-D-glucopyranosyl-L-ascorbic acid by a polymer catalyst in N,N-dimethylformamide.
Carbohydr. Res. 346(15) , 2511-4, (2011) 6-O-Dodecanoyl-2-O-α-D-glucopyranosyl-L-ascorbic acid (6-sDode-AA-2G) was synthesized from 2-O-α-D-glucopyranosyl-L-ascorbic acid and lauric anhydride with a polymer catalyst, poly(4-vinylpyridine), in N,N-dimethylformamide without the introduction of protect... |
|
|
Monoacylation of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid by protease in N,N-dimethylformamide with low water content.
Carbohydr. Res. 345(12) , 1658-62, (2010) 2-O-alpha-D-Glucopyranosyl-L-ascorbic acid (AA-2G) laurate was synthesized from AA-2G and vinyl laurate with a protease from Bacillus subtilis in N,N-dimethylformamide (DMF) with low water content. Addition of water to DMF dramatically enhanced monoacyl AA-2G... |
|
|
Signal transduction pathway for L-ascorbic acid- and L-ascorbic acid 2-glucoside-induced DNA synthesis and cell proliferation in primary cultures of adult rat hepatocytes.
Eur. J. Pharmacol. 683(1-3) , 276-84, (2012) We examined the effects of L-ascorbic acid and its analogues on DNA synthesis and cell proliferation. We also investigated the signal transduction pathways involved in the induction of mitogenesis by L-ascorbic acid and its analogues using primary cultures of... |
|
|
Bioavailability of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid as ascorbic acid in healthy humans.
Nutrition 25(6) , 686-91, (2009) 2-O-alpha-D-glucopyranosyl-L-ascorbic acid (AA2G) is a stable glycoside, but its conversion to bioavailable ascorbic acid (AsA) in humans remains unknown. The aim of this study was to clarify that AA2G is hydrolyzed by human intestinal maltase and AA2G by ora... |
|
|
Intramolecular acyl migration and enzymatic hydrolysis of a novel monoacylated ascorbic acid derivative, 6-O-dodecanoyl-2-O-alpha-d-glucopyranosyl-L-ascorbic acid.
Bioorg. Med. Chem. 18(16) , 6179-83, (2010) A stable ascorbic acid derivative, 2-O-alpha-D-glucopyranosyl-L-ascorbic acid (AA-2G), exhibits vitamin C activity in vitro and in vivo after enzymatic hydrolysis to ascorbic acid. AA-2G has been approved by the Japanese Government as a quasi-drug principal i... |
|
|
Stability studies of ascorbic acid 2-glucoside in cosmetic lotion using surface response methodology.
Bioorg. Med. Chem. Lett. 23(6) , 1583-7, (2013) Ascorbic acid 2-glucoside (AA-2G) has been widely used in cream and lotion types of cosmetic products. Thus, the degradation of AA-2G caused by the temperature change and pH variation was very critical for determining the bio-functionality of cosmetics. Respo... |
|
|
Protease-catalyzed monoacylation of 2-O-α-D-glucopyranosyl-L-ascorbic acid in three solvent systems.
Biosci. Biotechnol. Biochem. 74(9) , 1969-71, (2010) 6-O-dodecanoyl-2-O-α-D-glucopyranosyl-L-ascorbic acid (6-sDode-AA-2G) was synthesized from 2-O-α-D-glucopyranosyl-L-ascorbic acid and vinyl laurate with a protease from Bacillus subtilis in 30% dimethylformamide (DMF)/dioxane with a low water content. The add... |