Monoacylation of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid by protease in N,N-dimethylformamide with low water content.
Akihiro Tai, Tasuku Mori, Yuka Kimura, Hideyuki Ito
Index: Carbohydr. Res. 345(12) , 1658-62, (2010)
Full Text: HTML
Abstract
2-O-alpha-D-Glucopyranosyl-L-ascorbic acid (AA-2G) laurate was synthesized from AA-2G and vinyl laurate with a protease from Bacillus subtilis in N,N-dimethylformamide (DMF) with low water content. Addition of water to DMF dramatically enhanced monoacyl AA-2G synthesis. Maximum synthetic activity was observed when 3% (v/v) water was added to the reaction medium. Under the optimal reaction conditions, 5-O-dodecanoyl-2-O-alpha-D-glucopyranosyl-L-ascorbic acid, 2-O-(6'-O-dodecanoyl-alpha-D-glucopyranosyl)-L-ascorbic acid, and 6-O-dodecanoyl-2-O-alpha-D-glucopyranosyl-L-ascorbic acid were synthesized in yields of 5.5%, 3.2%, and 20.4%, respectively.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
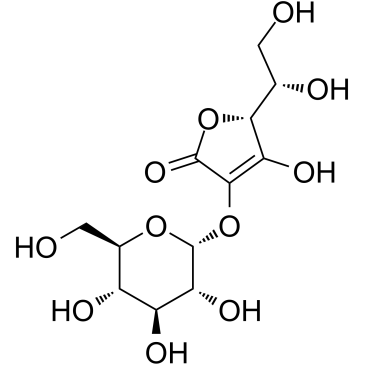 |
Ascorbyl Glucoside
CAS:129499-78-1 |
C12H18O11 |
|
Functions, applications and production of 2-O-D-glucopyranos...
2012-07-01 [Appl. Microbiol. Biotechnol. 95(2) , 313-20, (2012)] |
|
Systems engineering of tyrosine 195, tyrosine 260, and gluta...
2013-01-01 [Appl. Environ. Microbiol. 79(2) , 672-7, (2013)] |
|
Evaluation of the antioxidative capability of commonly used ...
2012-07-02 [J. Photochem. Photobiol. B, Biol. 112 , 7-15, (2012)] |
|
Regioselective monoacylation of 2-O-α-D-glucopyranosyl-L-asc...
2011-11-08 [Carbohydr. Res. 346(15) , 2511-4, (2011)] |
|
Signal transduction pathway for L-ascorbic acid- and L-ascor...
2012-05-15 [Eur. J. Pharmacol. 683(1-3) , 276-84, (2012)] |