H-Tic-OH
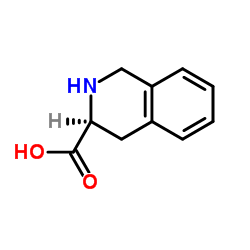
H-Tic-OH structure
|
Common Name | H-Tic-OH | ||
|---|---|---|---|---|
| CAS Number | 74163-81-8 | Molecular Weight | 177.20 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 372.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H11NO2 | Melting Point | 300ºC | |
| MSDS | Chinese USA | Flash Point | 178.8±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Acid catalysis in the formation of dioxopiperazines from peptides containing tetrahydroisoquinoline-3-carboxylic acid at position 2.
Int. J. Pept. Protein Res. 45(6) , 567-73, (1995) The kinetics of the spontaneous formation of 2,5-dioxopiperazines from peptides containing the Tic (1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) residue in the 2-position of the sequence has been studied in DMSO and water solution. The reaction is first ... |
|
|
2-Substituted (S)-2-(3,3-dimethyl-1-oxo-10,10a-dihydroimidazo[1,5-b]isoquinolin-2(1H,3H,5H)-yl)acetic acids: Conformational prediction, synthesis, anti-thrombotic and vasodilative evaluation.
Bioorg. Med. Chem. 19 , 871-82, (2011) (S)-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (TIC) can inhibit thrombosis by inhibiting platelet aggregation. The investigation of amino acids modified TIC reveals that a stretching conformation is critical for high anti-thrombotic activity. The confo... |
|
|
Constrained phenylalanine analogues. Preferred conformation of the 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) residue.
Int. J. Pept. Protein Res. 40 , 222, (1992) Three Tic-containing (Tic = 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) model peptides were synthesized to assess the tendency of this constrained Phe analogue to fold into a beta-bend and a helical structure, and to adopt a preferred side-chain disposi... |
|
|
2-Acyl-tetrahydroisoquinoline-3-carboxylic acids: lead compounds with triple actions, peroxisome proliferator-activated receptor α/γ agonist and protein-tyrosine phosphatase 1B inhibitory activities.
Chem. Pharm. Bull. 59(7) , 876-9, (2011) 2-Acyl-tetrahydroisoquinoline-3-carboxylic acid derivatives were synthesized and biologically evaluated. (S)-2-(2,4-Hexadienoyl)-7-[2-(5-methyl-2-phenyloxazol-4-yl)ethoxy]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (14) showed peroxisome proliferator-ac... |
|
|
A novel series of (S)-2,7-substituted-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids: peroxisome proliferator-activated receptor α/γ dual agonists with protein-tyrosine phosphatase 1B inhibitory activity.
Chem. Pharm. Bull. 59(10) , 1233-42, (2011) Novel 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid derivatives were synthesized and (S)-7-(2-{2-[(E)-2-cyclopentylvinyl]-5-methyloxazol-4-yl}ethoxy)-2-[(2E,4E)-hexadienoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (14c) was identified as a peroxis... |
|
|
Deletion of Ac-NMePhe(1) from [NMePhe(1) ]arodyn under acidic conditions, part 1: effects of cleavage conditions and N-terminal functionality.
Biopolymers 96(1) , 97-102, (2011) Peptides containing N-methylamino acids can exhibit improved pharmacodynamic and pharmacokinetic profiles compared to nonmethylated peptides, and therefore interest in these N-methylated peptides has been increasing in recent years. Arodyn (Ac[Phe¹,²,³,Arg⁴,D... |
|
|
Applications and modifications of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) in peptides and peptidomimetics design and discovery.
Curr. Protein Pept. Sci. 11(8) , 752-8, (2010) Tic, short for 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, is a kind of unnatural α-amino acids. Due to its distinct geometrical conformation and biological activity, the structure of Tic, regarded as the surrogate of proline and the rigid analogue of p... |
|
|
[TIC4]endomorphins, analogues of endomorphins, have significantly enhanced vasorelaxant effects in rat aorta rings.
Protein Pept. Lett. 12(4) , 323-6, (2005) [Tic(4)]EM1 and [Tic(4)]EM2, new endomorphins (EMs) analogues, caused relaxation of rat aorta rings precontracted with phenylphrine in a concentration-dependent manner and were 240- to 370-fold more potent than EMs. This effect was inhibited by endothelium re... |
|
|
Novel C-terminus modifications of the Dmt-Tic motif: a new class of dipeptide analogues showing altered pharmacological profiles toward the opioid receptors.
J. Med. Chem. 44(15) , 2387-90, (2001) The design, synthesis and pharmacological evaluation of a novel class of Dmt-Tic dipeptide analogues are described. These resulting analogues bearing different C-terminal functionalities were found to bind to the human delta receptor with high affinity. One s... |
|
|
An exploration of the effects of L- and D-tetrahydroisoquinoline-3-carboxylic acid substitutions at positions 2, 3 and 7 in cyclic and linear antagonists of vasopressin and oxytocin and at position 3 in arginine vasopressin.
J. Pept. Sci. 1(1) , 66-79, (1995) We have investigated the effects of mono-substitutions with the conformationally restricted amino acid, 1,2,3,4 tetrahydroisoquinoline-3-carboxylic acid (Tic) at position 3 in arginine vasopressin (AVP), at positions 2, 3 and 7 in potent non-selective cyclic ... |