Applications and modifications of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) in peptides and peptidomimetics design and discovery.
Yingjie Zhang, Hao Fang, Wenfang Xu
Index: Curr. Protein Pept. Sci. 11(8) , 752-8, (2010)
Full Text: HTML
Abstract
Tic, short for 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, is a kind of unnatural α-amino acids. Due to its distinct geometrical conformation and biological activity, the structure of Tic, regarded as the surrogate of proline and the rigid analogue of phenylalanine or tyrosine, has been introduced into many compounds, which target diverse enzymes or receptors. The most successful example is that substituting the Tic residue for the proline residue of enalapril led to an approved drug quinapril. In this review, we will summarize the applications and modifications of Tic in peptides and peptidomimetics design and discovery, and hope to spark medicinal researchers' inspiration in the field of protein and peptide drug design and optimization.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
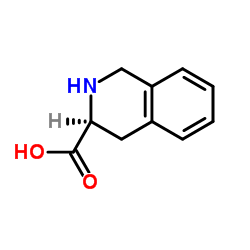 |
H-Tic-OH
CAS:74163-81-8 |
C10H11NO2 |
|
Acid catalysis in the formation of dioxopiperazines from pep...
1995-06-01 [Int. J. Pept. Protein Res. 45(6) , 567-73, (1995)] |
|
2-Substituted (S)-2-(3,3-dimethyl-1-oxo-10,10a-dihydroimidaz...
2011-01-15 [Bioorg. Med. Chem. 19 , 871-82, (2011)] |
|
Constrained phenylalanine analogues. Preferred conformation ...
1992-01-01 [Int. J. Pept. Protein Res. 40 , 222, (1992)] |
|
2-Acyl-tetrahydroisoquinoline-3-carboxylic acids: lead compo...
2011-01-01 [Chem. Pharm. Bull. 59(7) , 876-9, (2011)] |
|
A novel series of (S)-2,7-substituted-1,2,3,4-tetrahydroisoq...
2011-01-01 [Chem. Pharm. Bull. 59(10) , 1233-42, (2011)] |