3-Cyclohexenecarboxylic acid
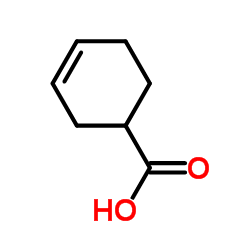
3-Cyclohexenecarboxylic acid structure
|
Common Name | 3-Cyclohexenecarboxylic acid | ||
|---|---|---|---|---|
| CAS Number | 4771-80-6 | Molecular Weight | 126.153 | |
| Density | 1.081 | Boiling Point | 238 ºC | |
| Molecular Formula | C7H10O2 | Melting Point | 17ºC | |
| MSDS | Chinese USA | Flash Point | 109.9±16.2 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs).
Environ. Sci. Technol. 48(4) , 2344-51, (2014) The effect of halides on organic contaminant destruction efficiency was compared for UV/H2O2 and UV/S2O8(2-) AOP treatments of saline waters; benzoic acid, 3-cyclohexene-1-carboxylic acid, and cyclohexanecarboxylic acid were used as models for aromatic, alken... |
|
|
Mutational biosynthesis of a FK506 analogue containing a non-natural starter unit.
Mol. Biosyst. 9(5) , 944-7, (2013) A FK506 analogue containing a non-natural starter unit was obtained through mutasynthesis by feeding cultures of Streptomyces sp. KCTC 11604BP fkbO deletion mutant with 3-cyclohexene-1-carboxylic acid. The structure of the new compound, 32-dehydroxy-FK506, an... |
|
|
Synthesis and antiviral evaluation of cis-substituted cyclohexenyl and cyclohexanyl nucleosides.
J. Med. Chem. 48(2) , 450-6, (2005) Starting from commercially available (rac)-3-cyclohexene-1-carboxylic acid, a series of purine and pyrimidine cis-substituted cyclohexenyl and cyclohexanyl nucleosides were synthesized through a key Mitsunobu reaction. Antiviral evaluations were performed on ... |
|
|
Experimental elicitation with hydroxyisohexyl-3-cyclohexene carboxaldehyde-containing deodorants.
Contact Dermatitis 56(3) , 146-50, (2007) Hydroxyisohexyl-3-cyclohexene carboxaldehyde (HICC) known as Lyral is a frequent allergen. It is used in more than 50% of marketed deodorants. The aim of the present study was to determine elicitation thresholds for HICC under simulated conditions of deodoran... |
|
|
The metabolism of cyclohexanecarboxylic acid and 3-cyclohexenecarboxylic acid by Pseudomonas putida.
Can. J. Microbiol. 28(12) , 1324-9, (1982) A strain of Pseudomonas putida grew rapidly on cyclohexanecarboxylic acid as a sole source of carbon. A CoA-mediated beta-oxidation pathway was induced for the metabolism of the compound. The organism could not utilize 3-cyclohexenecarboxylic acid as a sole s... |
|
|
Total synthesis of (.+-.)-methyl shikimate and (.+-.)-3-phosphoshikimic acid. Bartlett PA and McQuaid LA.
J. Am. Chem. Soc. 106(25) , 7854-60, (1984)
|
|
|
Bromination of 3-cyclohexene-1-carboxylic acid, epoxydation of methyl 3-cyclohexene-1-carboxylate and opening of methyl cis-and trans-3, 4-epoxycyclohexane-1-carboxylate: Stereochemical results. Bellucci G, et al.
Tetrahedron 28(13) , 3393-99, (1972)
|