Synthesis and antiviral evaluation of cis-substituted cyclohexenyl and cyclohexanyl nucleosides.
Karine Barral, Jérôme Courcambeck, Gérard Pèpe, Jan Balzarini, Johan Neyts, Erik De Clercq, Michel Camplo
Index: J. Med. Chem. 48(2) , 450-6, (2005)
Full Text: HTML
Abstract
Starting from commercially available (rac)-3-cyclohexene-1-carboxylic acid, a series of purine and pyrimidine cis-substituted cyclohexenyl and cyclohexanyl nucleosides were synthesized through a key Mitsunobu reaction. Antiviral evaluations were performed on HIV, coxsackie B3, and herpes viruses (HSV-1, HSV-2, VZV, HCMV). Three compounds showed moderate activity against HSV-1 and coxsackie viruses. Specific computer modeling studies were performed on HSV-1 thymidine kinase in order to understand the enzyme activation of an analogue showing moderate antiviral activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
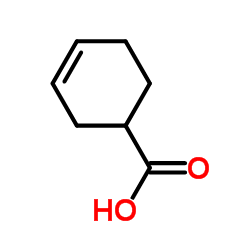 |
3-Cyclohexenecarboxylic acid
CAS:4771-80-6 |
C7H10O2 |
|
Comparison of halide impacts on the efficiency of contaminan...
2014-02-18 [Environ. Sci. Technol. 48(4) , 2344-51, (2014)] |
|
Mutational biosynthesis of a FK506 analogue containing a non...
2013-05-01 [Mol. Biosyst. 9(5) , 944-7, (2013)] |
|
Experimental elicitation with hydroxyisohexyl-3-cyclohexene ...
2007-03-01 [Contact Dermatitis 56(3) , 146-50, (2007)] |
|
The metabolism of cyclohexanecarboxylic acid and 3-cyclohexe...
1982-12-01 [Can. J. Microbiol. 28(12) , 1324-9, (1982)] |
|
Total synthesis of (.+-.)-methyl shikimate and (.+-.)-3-phos...
[J. Am. Chem. Soc. 106(25) , 7854-60, (1984)] |