5-Methylchrysene
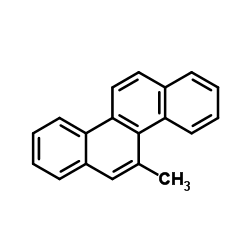
5-Methylchrysene structure
|
Common Name | 5-Methylchrysene | ||
|---|---|---|---|---|
| CAS Number | 3697-24-3 | Molecular Weight | 242.314 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 449.4±12.0 °C at 760 mmHg | |
| Molecular Formula | C19H14 | Melting Point | 117.5ºC | |
| MSDS | USA | Flash Point | 217.8±13.7 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
|
QSPR modeling of octanol/water partition coefficient for vitamins by optimal descriptors calculated with SMILES.
Eur. J. Med. Chem. 43 , 714-40, (2008) Simplified molecular input line entry system (SMILES) has been utilized in constructing quantitative structure-property relationships (QSPR) for octanol/water partition coefficient of vitamins and organic compounds of different classes by optimal descriptors.... |
|
|
Polycyclic aromatic hydrocarbons: 15 Listings - benz[a]anthracene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenz[a,h]acridine, dibenz[a,j]acridine, dibenz[a,h]anthracene, 7H-dibenzo[c,g]carbazole, dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, indeno[1,2,3-cd]pyrene, 5-methylchrysene.
Rep. Carcinog. 12 , 353-61, (2011)
|
|
|
TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation.
Neuron 79(6) , 1086-93, (2013) Dynamic changes in 5-methylcytosine (5mC) have been implicated in the regulation of gene expression critical for consolidation of memory. However, little is known about how these changes in 5mC are regulated in the adult brain. The enzyme methylcytosine dioxy... |
|
|
Analysis of carcinogenic polycyclic aromatic hydrocarbons in complex environmental mixtures by LC-APPI-MS/MS.
Anal. Chim. Acta 702(2) , 218-24, (2011) Here we developed a highly sensitive, fast and reliable liquid chromatography tandem mass spectrometry (LC-MS/MS) method for the detection and analysis of 16 different polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs that have been identified as carcino... |
|
|
Reactions of dihydrodiol epoxides of 5-methylchrysene and 5, 6-dimethylchrysene with DNA and deoxyribonucleotides.
Chem. Res. Toxicol. 12(4) , 347-52, (1999) Both syn and anti dihydrodiol epoxides from 5-methylchrysene (5-MCDE) and 5,6-dimethylchrysene (5,6-DMCDE) were reacted under the same conditions with native DNA, denatured DNA, and purine deoxyribonucleotides, and the products were quantified. The extents of... |
|
|
Tumor multiplicity, DNA adducts and K-ras mutation pattern of 5-methylchrysene in strain A/J mouse lung.
Carcinogenesis 15(11) , 2613-8, (1994) This study was undertaken to evaluate the carcinogenic potential of 5-methylchrysene (5-MeC) in strain A/J mouse lung and to correlate the 5-MeC-DNA adduct profile in lung tissue with the mutation spectrum in the K-ras gene of lung tumors. Strain A/J mice rec... |
|
|
Bay-region distortions in a methanol adduct of a bay-region diol epoxide of the carcinogen 5-methylchrysene.
Carcinogenesis 17(11) , 2507-11, (1996) The three-dimensional structure of the product of the reaction of a diol epoxide of the carcinogen 5-methylchrysene with methanol has been determined by an X-ray diffraction analysis. The diol epoxide used to obtain this compound contains a stereochemically h... |
|
|
Comparative metabolism and DNA binding of 6-nitro-5-methylchrysene and 5-methylchrysene.
Carcinogenesis 8(9) , 1327-31, (1987) The metabolic activation in mouse skin of the strong carcinogen, 5-methylchrysene (5-MeC) was compared to that of the inactive compound, 6-nitro-5-methylchrysene (6-NO2-5-MeC). Metabolites of 6-NO2-5-MeC, formed using rat liver homogenates, were identified ba... |
|
|
Metabolism of chrysene, 5-methylchrysene, 6-methylchrysene and 5,6-dimethylchrysene in rat liver cytosol, in vitro, and in rat subcutaneous tissue, in vivo.
Chem. Biol. Interact. 77(2) , 203-21, (1991) The polynuclear aromatic hydrocarbon chrysene undergoes a bioalkylation substitution reaction in vitro, in rat liver cytosol preparations, and in vivo, in rat dorsal subcutaneous tissue to yield 6-methylchrysene as a metabolite. In addition, both 5-methyl- an... |
|
|
Comparative metabolism of chrysene and 5-methylchrysene by rat and rainbow trout liver microsomes.
Toxicol. Sci. 72(2) , 260-6, (2003) We have investigated the metabolism of chrysene (CHR) and 5-methychyrsene (5-MeCHR) by Shasta rainbow trout (Oncorhyncus mykiss) and Long Evans rat liver microsomes to assess the effect of a non-benzo ring methyl substituent on the reactions involved in the m... |