| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Bromobenzene
CAS:108-86-1 |
|
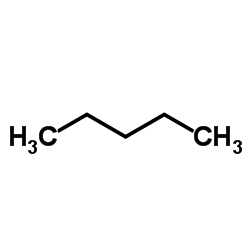 |
Pentane
CAS:109-66-0 |
|
 |
Triphenylene
CAS:217-59-4 |
|
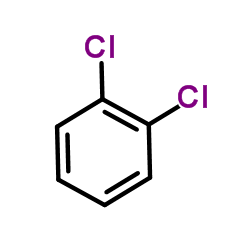 |
1,2-Dichlorobenzene
CAS:95-50-1 |
|
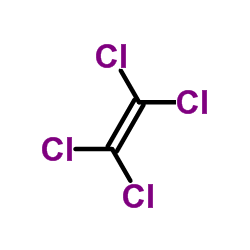 |
Tetrachloroethylene
CAS:127-18-4 |
|
 |
N-hexane
CAS:110-54-3 |
|
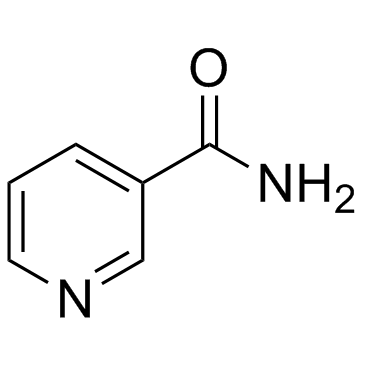 |
Nicotinamide
CAS:98-92-0 |
|
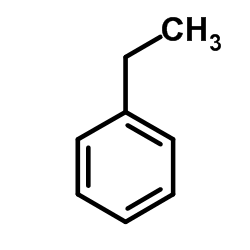 |
ether
CAS:100-41-4 |
|
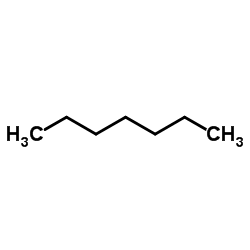 |
Heptane
CAS:142-82-5 |
|
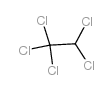 |
Ethane,1,1,1,2,2-pentachloro
CAS:76-01-7 |